当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cationic Rhodium(I)‐Catalyzed Carbonylative [2+2+1] Cycloaddition of Diynes
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-08-25 , DOI: 10.1002/ajoc.202000436 Tsumoru Morimoto 1 , JingWen Jia 2 , Yoshiko Yamaguchi 2 , Hiroki Tanimoto 2 , Kiyomi Kakiuchi 2
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-08-25 , DOI: 10.1002/ajoc.202000436 Tsumoru Morimoto 1 , JingWen Jia 2 , Yoshiko Yamaguchi 2 , Hiroki Tanimoto 2 , Kiyomi Kakiuchi 2
Affiliation
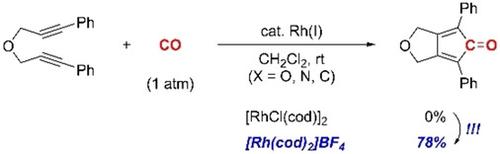
|
We describe Rh(I)‐catalyzed carbonylative [2+2+1] cycloaddition reactions of diynes with CO to give cyclopentadienone derivatives (CPDs). Previous reports on the use of neutral rhodium(I) complexes, such as [RhCl(cod)]2 and RhCl(PPh3)3, indicate that no CPD products are formed or that they are produced in low yields, even at elevated temperatures. The reaction described herein, which uses a cationic rhodium(I) complex, [Rh(cod)2]BF4, as a catalyst, proceeds smoothly at room temperature to give cyclopentadienone derivatives. The key to this is the use the cationic rhodium(I) complex as a catalyst, which produces an additional coordination site on the rhodium for the substrate (a diyne) to approach, than neutral rhodium complexes.
中文翻译:

阳离子铑(I)催化的二炔羰基化[2 + 2 + 1]环加成反应
我们描述了二炔与Rh的Rh(I)催化的羰基化[2 + 2 + 1]环加成反应,得到环戊二烯酮衍生物(CPDs)。以前有关使用中性铑(I)配合物(例如[RhCl(cod)] 2和RhCl(PPh 3)3)的报告表明,即使在高温下也不会形成CPD产品或以低收率生产它们。本文所述的反应,该反应使用阳离子铑(I)络合物[Rh(cod)2 ] BF 4,作为催化剂,在室温下平稳进行,得到环戊二烯酮衍生物。这样做的关键是使用阳离子铑(I)络合物作为催化剂,与中性铑络合物相比,它在铑上产生了一个额外的配位位,使底物(二炔)能够接近。
更新日期:2020-08-25
中文翻译:

阳离子铑(I)催化的二炔羰基化[2 + 2 + 1]环加成反应
我们描述了二炔与Rh的Rh(I)催化的羰基化[2 + 2 + 1]环加成反应,得到环戊二烯酮衍生物(CPDs)。以前有关使用中性铑(I)配合物(例如[RhCl(cod)] 2和RhCl(PPh 3)3)的报告表明,即使在高温下也不会形成CPD产品或以低收率生产它们。本文所述的反应,该反应使用阳离子铑(I)络合物[Rh(cod)2 ] BF 4,作为催化剂,在室温下平稳进行,得到环戊二烯酮衍生物。这样做的关键是使用阳离子铑(I)络合物作为催化剂,与中性铑络合物相比,它在铑上产生了一个额外的配位位,使底物(二炔)能够接近。











































 京公网安备 11010802027423号
京公网安备 11010802027423号