当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Decarboxylative Borylation of Stabilized and Activated Carbon Radicals.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-08-25 , DOI: 10.1002/anie.202008138 Qiang Zhang 1 , Xiaojuan Li 1 , Weigang Zhang 1 , Shengyang Ni 1 , Yi Wang 1 , Yi Pan 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-08-25 , DOI: 10.1002/anie.202008138 Qiang Zhang 1 , Xiaojuan Li 1 , Weigang Zhang 1 , Shengyang Ni 1 , Yi Wang 1 , Yi Pan 1
Affiliation
|
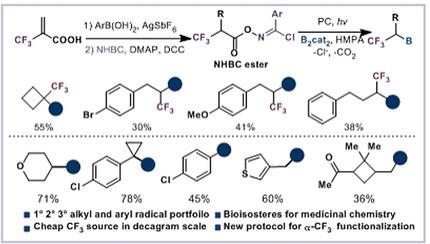
Redox‐active esters (RAEs) as active radical precursors have been extensively studied for C−B bond formations. However, the analogous transformations of stabilized radicals from the corresponding acid precursors remain challenging owing to the strong preference towards single‐electron oxidation to the stable carbocations. This work describes a general strategy for rapid access to various aliphatic and aromatic boronic esters by mild photoinduced decarboxylative borylation. Both aryl and alkyl radicals could be generated from the leaving group‐assisted N‐hydroxybenzimidoyl chloride esters, even α‐CF3 substituted substrates could be activated for further elaboration.
中文翻译:

稳定和活性碳自由基的脱羧硼化。
作为活性自由基前体的氧化还原活性酯(RAE)已针对C-B键的形成进行了广泛的研究。然而,由于强烈倾向于单电子氧化而不是稳定的碳正离子化,相应酸前体对稳定基团的类似转化仍然具有挑战性。这项工作描述了通过温和的光诱导脱羧硼化作用快速获得各种脂族和芳族硼酸酯的一般策略。芳基和烷基基团可以从离去基团辅助的N-羟基亚酰氯酯产生,即使α-CF 3取代的基材可以为进一步阐述被激活。
更新日期:2020-08-25
中文翻译:

稳定和活性碳自由基的脱羧硼化。
作为活性自由基前体的氧化还原活性酯(RAE)已针对C-B键的形成进行了广泛的研究。然而,由于强烈倾向于单电子氧化而不是稳定的碳正离子化,相应酸前体对稳定基团的类似转化仍然具有挑战性。这项工作描述了通过温和的光诱导脱羧硼化作用快速获得各种脂族和芳族硼酸酯的一般策略。芳基和烷基基团可以从离去基团辅助的N-羟基亚酰氯酯产生,即使α-CF 3取代的基材可以为进一步阐述被激活。











































 京公网安备 11010802027423号
京公网安备 11010802027423号