International Journal for Parasitology: Drugs and Drug Resistance ( IF 4.1 ) Pub Date : 2020-08-25 , DOI: 10.1016/j.ijpddr.2020.08.006 Kun Li 1 , Gregory M Grooms 2 , Shahbaz M Khan 1 , Anolan Garcia Hernandez 2 , William H Witola 1 , Jozef Stec 3
|
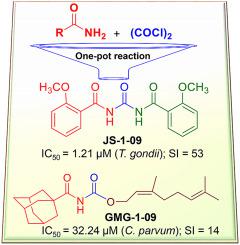
Toxoplasma gondii and Cryptosporidium parvum are protozoan parasites that are highly prevalent and opportunistically infect humans worldwide, but for which completely effective and safe medications are lacking. Herein, we synthesized a series of novel small molecules bearing the diacyl urea scaffold and related structures, and screened them for in vitro cytotoxicity and antiparasitic activity against T. gondii and C. parvum. We identified one compound (GMG-1-09), and four compounds (JS-1-09, JS-2-20, JS-2-35 and JS-2-49) with efficacy against C. parvum and T. gondii, respectively, at low micromolar concentrations and showed appreciable selectivity in human host cells. Among the four compounds with efficacy against T. gondii, JS-1-09 representing the diacyl urea scaffold was the most effective, with an anti-Toxoplasma IC50 concentration (1.21 μM) that was nearly 53-fold lower than its cytotoxicity IC50 concentration, indicating that this compound has a good selectivity index. The other three compounds (JS-2-20, JS-2-35 and JS-2-49) were structurally more divergent from JS-1-09 as they represent the acyl urea and acyl carbamate scaffold. This appeared to correlate with their anti-Toxoplasma activity, suggesting that these compounds’ potency can likely be enhanced by selective structural modifications. One compound, GMG-1-09 representing acyl carbamate scaffold, depicted in vitro efficacy against C. parvum with an IC50 concentration (32.24 μM) that was 14-fold lower than its cytotoxicity IC50 concentration in a human intestinal cell line. Together, our studies unveil a series of novel synthetic acyl/diacyl urea and acyl carbamate scaffold-based small molecule compounds with micromolar activity against T. gondii and C. parvum that can be explored further for the development of the much-needed novel anti-protozoal drugs.
中文翻译:

新型酰基氨基甲酸酯和酰基/二酰基脲显示出对弓形虫和小隐孢子虫的体外功效
弓形虫和隐孢子虫是原生动物寄生虫,在全世界范围内非常流行并伺机感染人类,但目前缺乏完全有效和安全的药物。在此,我们合成了一系列带有二酰基脲支架和相关结构的新型小分子,并筛选了它们对弓形虫和微小弯曲菌的体外细胞毒性和抗寄生虫活性。我们鉴定出一种化合物 (GMG-1-09) 和四种化合物 (JS-1-09、JS-2-20、JS-2-35 和 JS-2-49) 对微小念珠菌和弓形虫有效分别在低微摩尔浓度下,并且在人类宿主细胞中表现出明显的选择性。在四种对弓形虫有效的化合物中,代表二酰脲支架的JS-1-09是最有效的,其抗弓形虫IC 50浓度(1.21 μM)比其细胞毒性IC 50低近53倍。浓度,表明该化合物具有良好的选择性指数。其他三种化合物(JS-2-20、JS-2-35 和 JS-2-49)在结构上与 JS-1-09 的差异更大,因为它们代表酰基脲和酰基氨基甲酸酯支架。这似乎与其抗弓形虫活性相关,表明这些化合物的效力可能通过选择性结构修饰而增强。一种化合物,GMG-1-09,代表酰基氨基甲酸酯支架,描述了对微小念珠菌的体外功效,IC 50浓度(32.24 μM),比人肠细胞系中的细胞毒性 IC 50浓度低 14 倍。总之,我们的研究揭示了一系列新型合成酰基/二酰基脲和酰基氨基甲酸酯支架小分子化合物,这些化合物具有微摩尔抗弓形虫和微小弯曲菌活性,可进一步探索开发急需的新型抗弓形虫药物。原虫类药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号