Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-08-25 , DOI: 10.1016/j.tetlet.2020.152388 Rui Yuan , Ming-qi Li , Hang Zhou , Ya-wen Sun , Yan-ni Liang , Hui Xu , Yu Wan , Hui Wu
|
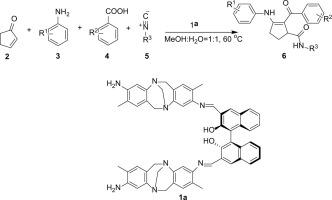
The 1,4-addition Ugi reaction can provide complex, novel and bioactive structures. However, Ugi reaction involving the 1,4-addition process has rarely been reported due to the Michael addition of amine to α,β-unsaturated ketones or aldehydes prevents the formation of the key intermediate iminium ion. In this paper, a Schiff base catalyst derived from Tröger's base-NH2 and (R)-BINOL-CHO was used successfully to promote the four-component 1,4-addition Ugi reaction of α,β-unsaturated cyclic ketones, carboxylic acids, aromatic amines and isocyanides to afford an α,β-keto amide 2-arylformyl-3-(arylamino)cyclopent-2-enecarboxamides in high yield (6, 83–96%) under mild condition. A variety of aromatic amines participated in the reaction efficiently, which showed the high efficiency of the catalyst. A reasonable catalysis and formation mechanism were come up with.
中文翻译:

源自Tröger碱和BINOL的Schiff碱催化四组分1,4-加成Ugi反应
1,4-加成的Ugi反应可以提供复杂,新颖和生物活性的结构。然而,由于胺向α,β-不饱和酮或醛的迈克尔加成,阻止了关键的中间体亚胺离子的形成,因此很少报道涉及1,4-加成过程的Ugi反应。本文成功地使用了由Tröger的碱式NH 2和(R)-BINOL-CHO衍生的席夫碱催化剂来促进α,β-不饱和环酮,羧酸的四组分1,4-加成Ugi反应,芳族胺和异氰化物得到α,β酮酰胺2- arylformyl -3-(芳基氨基)环戊-2- enecarboxamides以高收率(6,83-96%)在温和条件下。各种芳香胺有效地参与了反应,这表明催化剂的效率很高。提出了合理的催化和形成机理。










































 京公网安备 11010802027423号
京公网安备 11010802027423号