Parasitology International ( IF 1.5 ) Pub Date : 2020-08-25 , DOI: 10.1016/j.parint.2020.102179 Eri H Hayakawa 1 , Hirotomo Kato 1 , Glenn A Nardone 2 , Jiro Usukura 3
|
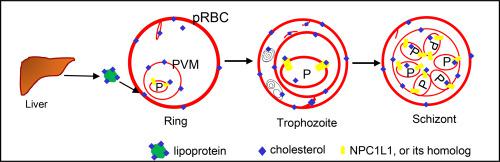
Plasmodium falciparum (P. falciparum) parasites still cause lethal infections worldwide, especially in Africa (https://www.who.int/publications/i/item/world-malaria-report-2019). During P. falciparum blood-stage infections in humans, low-density lipoprotein, high-density lipoprotein and cholesterol levels in the blood become low. Because P. falciparum lacks a de novo cholesterol synthesis pathway, it must import cholesterol from the surrounding environment. However, the origin of the cholesterol and how it is taken up by the parasite across the multiple membranes that surround it is not fully understood. To answer this, we used a cholesterol synthesis inhibiter (simvastatin), a cholesterol transport inhibitor (ezetimibe), and an activating ligand of the peroxisome proliferator-activated receptor α, called ciprofibrate, to investigate the effects of these agents on the intraerythrocytic growth of P. falciparum, both with and without HepG2 cells as the lipoprotein feeders. P. falciparum growth was inhibited in the presence of ezetimibe, but ezetimibe was not very effective at inhibiting P. falciparum growth when used in the co-culture system, unlike simvastatin, which strongly promoted parasite growth in this system. Ezetimibe is known to inhibit cholesterol absorption by blocking the activity of Niemann-Pick C1 like 1 (NPC1L1) protein, and simvastatin is known to enhance NPC1L1 expression in the human body's small intestine. Collectively, our results support the possibility that cholesterol import by P. falciparum involves hepatocytes, and cholesterol uptake into the parasite occurs via NPC1L1 protein or an NPC1L1 homolog during the erythrocytic stages of the P. falciparum lifecycle.
中文翻译:

恶性疟原虫感染的红细胞与HepG2细胞共培养的胆固醇摄取的前瞻性机制和来源。
恶性疟原虫(P.falciparum)寄生虫仍在全世界造成致命感染,尤其是在非洲(https://www.who.int/publications/i/item/world-malaria-report-2019)。在人类恶性疟原虫的血液阶段感染期间,血液中的低密度脂蛋白,高密度脂蛋白和胆固醇水平降低。因为恶性疟原虫缺乏从头胆固醇合成的途径,它必须从周围环境中导入胆固醇。但是,胆固醇的起源以及寄生虫如何通过其周围的多个膜吸收胆固醇的方式尚未完全了解。为了回答这个问题,我们使用了胆固醇合成抑制剂(辛伐他汀),胆固醇转运抑制剂(依泽替米贝)和过氧化物酶体增殖物激活受体α的活化配体ciprofibrate,研究了这些药物对大鼠红细胞生成的影响。恶性疟原虫,有或没有HepG2细胞作为脂蛋白饲养者。依泽替米贝的存在可抑制恶性疟原虫的生长,但是依泽替米贝对恶性疟原虫的抑制作用不是很有效与辛伐他汀不同,在共培养系统中使用时生长迅速,而辛伐他汀则强烈促进该系统中的寄生虫生长。已知依泽替米贝可通过阻断Niemann-Pick C1样1(NPC1L1)蛋白的活性来抑制胆固醇的吸收,而辛伐他汀则可增强NPC1L1在人体小肠中的表达。总的来说,我们的结果支持恶性疟原虫从胆固醇输入的胆固醇涉及肝细胞的可能性,并且在恶性疟原虫生命周期的红细胞生成阶段,胆固醇通过NPC1L1蛋白或NPC1L1同源物被寄生虫吸收。











































 京公网安备 11010802027423号
京公网安备 11010802027423号