European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-08-25 , DOI: 10.1016/j.ejmech.2020.112707 Hajjaj H M Abdu-Allah,Shang-Chuen Wu,Chun-Hung Lin,Yu-Yao Tseng
|
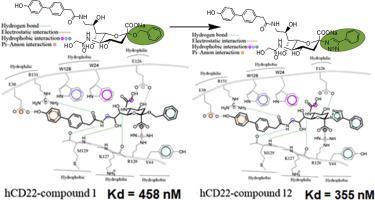
Ligand 1 was the first reported example of monomeric high-affinity synthetic CD22 ligand that regulated B cell activation in vitro, augmented antibody production and regulated immune responses in mice. Replacing O-glycoside linkage of 1 by nitrogen of triazole by click reaction afforded compounds which are as potent as the parent compound. The synthesis of the new compounds is straightforward with fewer synthetic steps and higher yield. Such a strategy provided stable ligand that can bind avidly and can be conjugated to drugs for B-cell targeting or multimeric formation. The new compounds were screened for their affinity to CD22, using surface plasmon resonance (SPR). Compound 12 was obtained as a bioisosteric analogue and an anomerically stable imitation of 1. It was, also, screened for MAG to test for selectivity and analyzed by molecular docking and dynamic simulation to explore the potential binding modes and source of selectivity within CD22. Our results could enable the development of small molecule drug capable of modulating the activity of CD22 in autoimmune diseases and malignancies derived from B-cells.
中文翻译:

设计,合成和分子对接研究作为不可水解的有效CD22配体的α-三唑基唾液酸苷。
配体1是第一个报道的单体高亲和力合成CD22配体的例子,该配体在体外调节B细胞活化,增加抗体产生并调节小鼠的免疫应答。更换ø糖苷的连杆1通过点击反应通过三唑氮,得到其是作为有效的作为母体化合物的化合物。新化合物的合成非常简单,合成步骤更少,产率更高。这种策略提供了稳定的配体,该配体可以结合并可以与B细胞靶向或多聚体形成药物结合。使用表面等离子体共振(SPR)筛选了新化合物对CD22的亲和力。化合物12获得作为生物电子等排类似物和的端基异构体稳定仿1。还对MAG进行筛选以测试选择性,并通过分子对接和动态模拟进行分析,以探索CD22中潜在的结合模式和选择性来源。我们的结果可以开发能够调节CD22在自身免疫性疾病和B细胞来源的恶性肿瘤中的活性的小分子药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号