当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereodivergent synthesis of dispiroheterocyclic structures comprising pyrrolidinyloxindole and imidazothiazolotriazine moieties.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-08-24 , DOI: 10.1039/d0ob01628d Alexei N Izmest'ev 1 , Galina A Gazieva , Valentina A Karnoukhova , Angelina N Kravchenko
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-08-24 , DOI: 10.1039/d0ob01628d Alexei N Izmest'ev 1 , Galina A Gazieva , Valentina A Karnoukhova , Angelina N Kravchenko
Affiliation
|
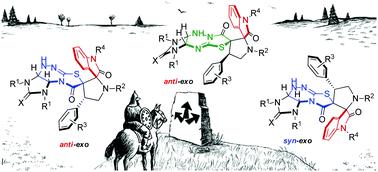
Highly diastereoselective methods for the synthesis of two different diastereomers of polynuclear dispiroheterocyclic compounds with five chiral centers comprising pyrrolidinyloxindole and imidazothiazolotriazine moieties (dispiro[imidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine-7,3′-pyrrolidine-2′,3′′-indoles]) have been developed on the basis of a dipolar cycloaddition of azomethine ylides to benzylidene derivatives of imidazothiazolotriazines and an alkali-induced rearrangement of the thiazolotriazine fragment. The different sequence of the cycloaddition and rearrangement stages allows us to perform the targeted synthesis of two diastereomerically pure products from the same starting compounds.
中文翻译:

包含吡咯烷基吲哚和咪唑并噻唑三嗪部分的双螺杂环结构的非对映发散合成。
用于合成具有五个手性中心的多核二螺杂环化合物的两种不同非对映异构体的高非对映选择性方法,包括吡咯烷基吲哚和咪唑并噻唑三嗪部分(二螺[咪唑并[4,5 -e ]噻唑并[2,3 - c ]-1,2,4-三嗪-7,3'-pyrrolidine-2',3''-indoles]) 是在偶极环加成偶氮甲碱叶立德与咪唑并噻唑三嗪的亚苄基衍生物和碱诱导的噻唑并三嗪片段重排的基础上开发的。环加成和重排阶段的不同顺序使我们能够从相同的起始化合物有针对性地合成两种非对映体纯产物。
更新日期:2020-09-16
中文翻译:

包含吡咯烷基吲哚和咪唑并噻唑三嗪部分的双螺杂环结构的非对映发散合成。
用于合成具有五个手性中心的多核二螺杂环化合物的两种不同非对映异构体的高非对映选择性方法,包括吡咯烷基吲哚和咪唑并噻唑三嗪部分(二螺[咪唑并[4,5 -e ]噻唑并[2,3 - c ]-1,2,4-三嗪-7,3'-pyrrolidine-2',3''-indoles]) 是在偶极环加成偶氮甲碱叶立德与咪唑并噻唑三嗪的亚苄基衍生物和碱诱导的噻唑并三嗪片段重排的基础上开发的。环加成和重排阶段的不同顺序使我们能够从相同的起始化合物有针对性地合成两种非对映体纯产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号