当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On the Stability and Conformational Dynamics of Cytochrome c in Ammonium Ionic Liquids.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-08-24 , DOI: 10.1021/acs.jpcb.0c05633 Ashok Pabbathi 1 , Anunay Samanta 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-08-24 , DOI: 10.1021/acs.jpcb.0c05633 Ashok Pabbathi 1 , Anunay Samanta 1
Affiliation
|
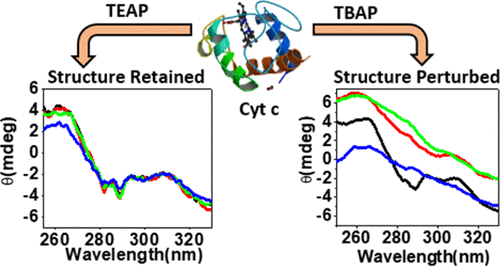
Owing to their potential applications in the extraction, purification, and preservation of biomolecules and biocatalysis, ionic liquids (ILs) have gained great attention in biotechnology. Although it is known that the structure and dynamics of proteins in ILs depend on the nature of both proteins and ILs, the biophysical mechanism governing the protein–IL interaction, which determines the stability of proteins or the activity of an enzyme in these nonconventional media, is yet to be understood clearly. Herein, we study the effect of two ammonium ILs, triethylammonium dihydrogen phosphate (TEAP) and tributylammonium dihydrogen phosphate (TBAP), on the stability and conformational dynamics of cytochrome c (Cyt c) in its native and unfolded states, employing primarily the single molecule-based fluorescence correlation spectroscopy (FCS) technique. The results show that the native structure of Cyt c is not significantly altered by TEAP, but the tertiary structure is perturbed to a great extent by TBAP, which comprises a longer alkyl chain. Fluctuations of the fluorescence intensity of Alexa488 dye-labeled Cyt c in FCS measurements reveal conformational dynamics (67 ± 10 μs) in the native state of Cyt c that is accelerated in the presence of both ILs but not affected when Cyt c is in its unfolded state. The present findings demonstrate how the stability of this protein can be modulated by using ammonium ILs of different alkyl chain lengths.
中文翻译:

铵离子液体中细胞色素c的稳定性和构象动力学
由于其在生物分子的提取,纯化和保存以及生物催化中的潜在应用,离子液体(ILs)在生物技术领域引起了极大的关注。尽管已知IL中蛋白质的结构和动力学取决于蛋白质和IL的性质,但是决定蛋白质与IL相互作用的生物物理机制决定了蛋白质在这些非常规介质中的稳定性或酶的活性,尚不清楚。在这里,我们研究了两种铵离子分子,三乙基磷酸二氢铵(TEAP)和三丁基磷酸二氢铵(TBAP)对细胞色素c(Cyt c)的稳定性和构象动力学的影响。)处于原始和未折叠状态,主要采用基于单分子的荧光相关光谱(FCS)技术。结果表明,Cyt c的天然结构不会被TEAP显着改变,但三级结构会受到包含更长烷基链的TBAP的极大干扰。FCS测量中Alexa488染料标记的Cyt c荧光强度的波动揭示了Cyt c天然状态的构象动力学(67±10μs),在两个IL均存在时会加速,但在Cyt c展开时不受影响州。目前的发现证明了如何通过使用不同烷基链长度的铵离子液体来调节这种蛋白质的稳定性。
更新日期:2020-09-18
中文翻译:

铵离子液体中细胞色素c的稳定性和构象动力学
由于其在生物分子的提取,纯化和保存以及生物催化中的潜在应用,离子液体(ILs)在生物技术领域引起了极大的关注。尽管已知IL中蛋白质的结构和动力学取决于蛋白质和IL的性质,但是决定蛋白质与IL相互作用的生物物理机制决定了蛋白质在这些非常规介质中的稳定性或酶的活性,尚不清楚。在这里,我们研究了两种铵离子分子,三乙基磷酸二氢铵(TEAP)和三丁基磷酸二氢铵(TBAP)对细胞色素c(Cyt c)的稳定性和构象动力学的影响。)处于原始和未折叠状态,主要采用基于单分子的荧光相关光谱(FCS)技术。结果表明,Cyt c的天然结构不会被TEAP显着改变,但三级结构会受到包含更长烷基链的TBAP的极大干扰。FCS测量中Alexa488染料标记的Cyt c荧光强度的波动揭示了Cyt c天然状态的构象动力学(67±10μs),在两个IL均存在时会加速,但在Cyt c展开时不受影响州。目前的发现证明了如何通过使用不同烷基链长度的铵离子液体来调节这种蛋白质的稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号