当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Atomic-Scale Structure and Catalysis on Positively Charged Bimetallic Sites for Generation of H2.
Nano Letters ( IF 9.6 ) Pub Date : 2020-08-24 , DOI: 10.1021/acs.nanolett.0c00852 Yu Tang 1 , Shiran Zhang 1 , Takat B Rawal 2 , Luan Nguyen 1 , Yasuhiro Iwasawa 3 , Shree R Acharya 2 , Jingyue Liu 4 , Sampyo Hong 2, 5 , Talat S Rahman 2, 6 , Franklin Tao 1
Nano Letters ( IF 9.6 ) Pub Date : 2020-08-24 , DOI: 10.1021/acs.nanolett.0c00852 Yu Tang 1 , Shiran Zhang 1 , Takat B Rawal 2 , Luan Nguyen 1 , Yasuhiro Iwasawa 3 , Shree R Acharya 2 , Jingyue Liu 4 , Sampyo Hong 2, 5 , Talat S Rahman 2, 6 , Franklin Tao 1
Affiliation
|
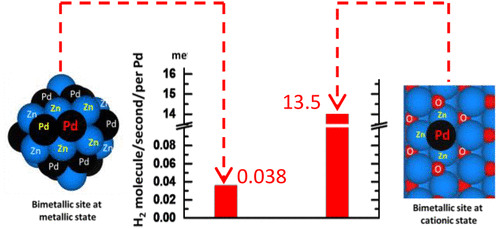
Here, we report that a cationic bimetallic site consisting of one Pd and three Zn atoms (Pd1Zn3) supported on ZnO (Pd1Zn3/ZnO) exhibits an extraordinarily high catalytic activity for the generation of H2 through methanol partial oxidation (MPO) that is 2–3 orders of magnitude higher than that of a metallic Pd–Zn site on Pd–Zn nanoalloy (Pd–Zn/ZnO). Computational studies uncovered that the positively charged Pd atom of the subnanometer Pd1Zn3 bimetallic site largely decreases the activation barrier for dehydrogenation of methanol as compared to a metallic Pd atom of Pd–Zn alloy, thus switching the rate-determining step of MPO from methanol dehydrogenation over a Pd–Zn alloy with high barrier to the O2 dissociation step on a cationic Pd1Zn3 site with a low barrier, which is supported by our kinetics studies. The significantly higher catalytic activity and selectivity for H2 production over a cationic bimetallic site suggest a new approach to design bimetallic catalysts.
中文翻译:

原子尺度结构和带正电荷的双金属位点催化生成H2。
在此,我们报道了一个由一个Pd和三个Zn原子(Pd 1 Zn 3)负载在ZnO(Pd 1 Zn 3 / ZnO)上的阳离子双金属位点,对于通过甲醇部分氧化生成H 2表现出极高的催化活性。(MPO)比Pd-Zn纳米合金(Pd-Zn / ZnO)上的金属Pd-Zn位置高2–3个数量级。计算研究发现,亚纳米级Pd 1 Zn 3的带正电的Pd原子与Pd-Zn合金中的金属Pd原子相比,双金属位点大大降低了甲醇脱氢的活化势垒,因此将MPO的速率确定步骤切换为对O 2的解离具有高势垒的Pd-Zn合金进行甲醇脱氢我们在动力学研究方面的支持下,在具有低势垒的阳离子Pd 1 Zn 3位点上进行了一步反应。在阳离子双金属位点上显着更高的催化活性和H 2生产的选择性表明了一种设计双金属催化剂的新方法。
更新日期:2020-09-10
中文翻译:

原子尺度结构和带正电荷的双金属位点催化生成H2。
在此,我们报道了一个由一个Pd和三个Zn原子(Pd 1 Zn 3)负载在ZnO(Pd 1 Zn 3 / ZnO)上的阳离子双金属位点,对于通过甲醇部分氧化生成H 2表现出极高的催化活性。(MPO)比Pd-Zn纳米合金(Pd-Zn / ZnO)上的金属Pd-Zn位置高2–3个数量级。计算研究发现,亚纳米级Pd 1 Zn 3的带正电的Pd原子与Pd-Zn合金中的金属Pd原子相比,双金属位点大大降低了甲醇脱氢的活化势垒,因此将MPO的速率确定步骤切换为对O 2的解离具有高势垒的Pd-Zn合金进行甲醇脱氢我们在动力学研究方面的支持下,在具有低势垒的阳离子Pd 1 Zn 3位点上进行了一步反应。在阳离子双金属位点上显着更高的催化活性和H 2生产的选择性表明了一种设计双金属催化剂的新方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号