当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct Conversion of N-Alkylamines to N-Propargylamines Through C–H Activation Promoted by Lewis Acid/Organocopper Catalysis: Application to Late-Stage Functionalization of Bioactive Molecules
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-08-24 , DOI: 10.1021/jacs.0c08599 Jessica Z Chan 1 , Ahmet Yesilcimen 1 , Min Cao 1 , Yuyang Zhang 1 , Bochao Zhang 1 , Masayuki Wasa 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-08-24 , DOI: 10.1021/jacs.0c08599 Jessica Z Chan 1 , Ahmet Yesilcimen 1 , Min Cao 1 , Yuyang Zhang 1 , Bochao Zhang 1 , Masayuki Wasa 1
Affiliation
|
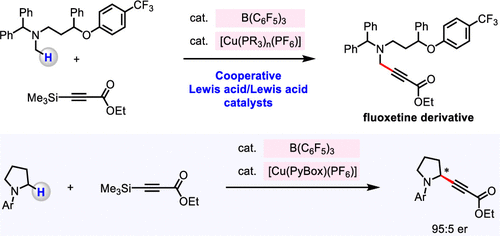
An efficient catalytic method to convert an alpha-C-H bond of N-alkylamines into an alpha-C-alkynyl bond was developed. In the past, such transformations were carried out under oxidative conditions, and the enantioselective variants were confined to tetrahydroisoquinoline derivatives. Here, we disclose a method for union of N-alkylamines and trimethylsilyl alkynes, without the presence of an external oxidant, and promoted through cooperative actions of two Lewis acids, B(C6F5)3 and a Cu-based complex. A variety of propargylamines can be synthesized in high diastereo- and enantioselectivity. The utility of the approach is demonstrated by late-stage site-selective modification of bioactive amines. Kinetic investigations that shed light on various mechanistic nuances of the catalytic process are presented.
中文翻译:

通过路易斯酸/有机铜催化促进的 C-H 活化将 N-烷基胺直接转化为 N-丙炔胺:在生物活性分子后期功能化中的应用
开发了一种将 N-烷基胺的 α-CH 键转化为 α-C-炔基键的有效催化方法。过去,此类转化是在氧化条件下进行的,对映选择性变体仅限于四氢异喹啉衍生物。在这里,我们公开了一种在不存在外部氧化剂的情况下,通过两种路易斯酸B(C6F5)3和Cu基络合物的协同作用促进N-烷基胺和三甲基甲硅烷基炔的结合的方法。可以以高非对映选择性和对映选择性合成多种炔丙胺。该方法的实用性通过生物活性胺的后期位点选择性修饰得到证明。动力学研究揭示了催化过程的各种机制细微差别。
更新日期:2020-08-24
中文翻译:

通过路易斯酸/有机铜催化促进的 C-H 活化将 N-烷基胺直接转化为 N-丙炔胺:在生物活性分子后期功能化中的应用
开发了一种将 N-烷基胺的 α-CH 键转化为 α-C-炔基键的有效催化方法。过去,此类转化是在氧化条件下进行的,对映选择性变体仅限于四氢异喹啉衍生物。在这里,我们公开了一种在不存在外部氧化剂的情况下,通过两种路易斯酸B(C6F5)3和Cu基络合物的协同作用促进N-烷基胺和三甲基甲硅烷基炔的结合的方法。可以以高非对映选择性和对映选择性合成多种炔丙胺。该方法的实用性通过生物活性胺的后期位点选择性修饰得到证明。动力学研究揭示了催化过程的各种机制细微差别。











































 京公网安备 11010802027423号
京公网安备 11010802027423号