当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Degradation of Ampicillin and Flucloxacillin Antibiotics via Oxidation by Alkaline Hexacyanoferrate(III): Kinetics and Mechanistic Aspects
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-08-24 , DOI: 10.1021/acs.iecr.0c03956 Ahmed Fawzy 1, 2 , Metwally Abdallah 1, 3 , Nada Alqarni 1, 4
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-08-24 , DOI: 10.1021/acs.iecr.0c03956 Ahmed Fawzy 1, 2 , Metwally Abdallah 1, 3 , Nada Alqarni 1, 4
Affiliation
|
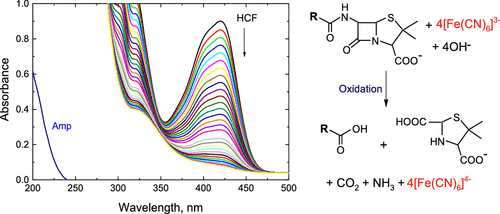
The kinetics and mechanistic aspects of the oxidation of two beta-lactam antibiotics (A), ampicillin and flucloxacillin, by alkaline hexacyanoferrate(III) (HCF(III)) were examined using spectrophotometry at a fixed temperature. The oxidation reactions showed a 1:4 (A: HCF(III)) stoichiometry. The reaction kinetics were found to follow first-order dependence for the oxidant and fractional first-order dependence for [A] and [OH–]. The enhancement of the ionic strength and dielectric constant was found to increase the oxidation rates. Free radical tests of the reactions showed positive results. The addition of HCF(II) as a predicted oxidation product did not considerably change the oxidation rates. Under the same experimental conditions, the oxidation rate of ampicillin was found to be slightly lower than that of flucloxacillin. The acquired oxidation products were recognized using spot testing and Fourier transform infrared spectra. A conceivable oxidation mechanism was suggested. A derived rate law expression was found to be consistent with the experimental results. The activation parameters were assessed and discussed.
中文翻译:

碱性十六烷基高铁酸盐(III)氧化降解氨苄青霉素和氟氯西林抗生素的动力学和机理
在固定温度下使用分光光度法检查了碱性六氰合铁酸盐(III)(HCF(III))氧化两种β-内酰胺抗生素(A)氨苄青霉素和氟氯西林的动力学和机理。氧化反应显示出1:4(A:HCF(III))化学计量。发现反应动力学遵循氧化剂的一阶依赖关系,[A]和[OH –]。发现提高离子强度和介电常数可提高氧化速率。反应的自由基测试显示出阳性结果。加入HCF(II)作为预计的氧化产物不会明显改变氧化速率。在相同的实验条件下,发现氨苄青霉素的氧化速率略低于氟氯西林。使用斑点测试和傅立叶变换红外光谱识别获得的氧化产物。提出了一种可能的氧化机理。发现派生的速率定律表达与实验结果一致。评估并讨论了激活参数。
更新日期:2020-09-16
中文翻译:

碱性十六烷基高铁酸盐(III)氧化降解氨苄青霉素和氟氯西林抗生素的动力学和机理
在固定温度下使用分光光度法检查了碱性六氰合铁酸盐(III)(HCF(III))氧化两种β-内酰胺抗生素(A)氨苄青霉素和氟氯西林的动力学和机理。氧化反应显示出1:4(A:HCF(III))化学计量。发现反应动力学遵循氧化剂的一阶依赖关系,[A]和[OH –]。发现提高离子强度和介电常数可提高氧化速率。反应的自由基测试显示出阳性结果。加入HCF(II)作为预计的氧化产物不会明显改变氧化速率。在相同的实验条件下,发现氨苄青霉素的氧化速率略低于氟氯西林。使用斑点测试和傅立叶变换红外光谱识别获得的氧化产物。提出了一种可能的氧化机理。发现派生的速率定律表达与实验结果一致。评估并讨论了激活参数。











































 京公网安备 11010802027423号
京公网安备 11010802027423号