当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gold at crossroads of radical generation and scavenging at density functional theory level: Nitrogen and oxygen free radicals versus their precursors in the face of nanogold
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-08-24 , DOI: 10.1002/poc.4126 Aliakbar Ahmadi 1 , Mohamad Z. Kassaee 1 , Mojgan Ayoubi‐Chianeh 1 , Alireza Fattahi 2
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-08-24 , DOI: 10.1002/poc.4126 Aliakbar Ahmadi 1 , Mohamad Z. Kassaee 1 , Mojgan Ayoubi‐Chianeh 1 , Alireza Fattahi 2
Affiliation
|
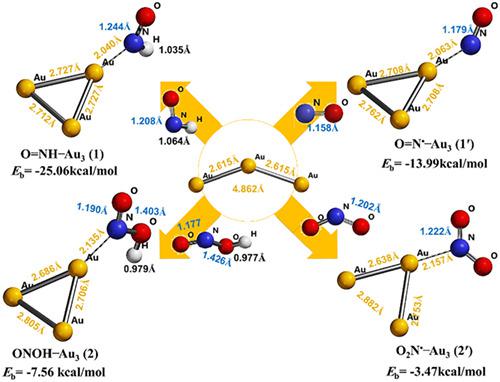
In our previous report (J. Phys. Org. Chem., 2017), we discussed the dual behavior of gold nanocluster (Au3 NC), where it scavenged reactive oxygen species (ROS) while promoted their generation to a lesser extent. Continuing this quest, we investigate the effects of Au3 NC on common reactive nitrogen species (RNS: ON˙ and ON˙O) and their precursors (ONH and ONOH, respectively), at B3LYP/LACVP+* level of theory. We compare the results with those of prevalent ROS (HO˙ and HOO˙) and their precursors (HOH and HOOH, respectively). To this end, various parameters are probed such as binding energy (Eb), bond dissociation energy (BDE), bond lengths, Mullikan spin density (MSD), critical point (CP) eigenvalues (atoms in molecules [AIM] analysis), and natural bond orbital (NBO) analysis including natural population analysis (NPA) and change of charge transfer energy (ΔECT). Our results show that in the presence of Au3 NC, BDEs of the NH and OH bonds increase in both ONHAu3 (1) and ONOHAu3 (2) compared with their free forms (ONH and ONOH, respectively). In other words, the tendency to dissociate these bonds and form their corresponding RNS (1′ and 2′) decreases in the presence of gold. This is exactly the opposite of what is observed for the OH bonds in HOHAu3 (3), HOOHAu3 (4), and their corresponding ROS (3′ and 4′). Therefore, we can conclude that gold shows a different behavior in the face of RNS compared with ROS where it is a better scavenger of ROS than RNS.
中文翻译:

处于自由基生成和清除的密度泛函理论水平的十字路口的黄金:面对纳米金,氮和氧自由基与它们的前体
在我们之前的报告(J. Phys。Org。Chem。,2017)中,我们讨论了金纳米团簇(Au 3 NC)的双重行为,该团簇清除了活性氧(ROS)并在较小程度上促进了它们的生成。继续这个任务,我们研究金的影响,3 NC上常见的活性氮类(RNS:ON˙和ON˙ O)及其前体(ON H和ON Ò H,分别在理论上达到B3LYP / LACVP + *水平。我们比较结果与那些流行的ROS(H 氧,氢 Ò O)及其前体(H Ò H和H ø Ò H,分别地)。为此,探索了各种参数,例如结合能(E b),键解离能(BDE),键长,穆利坎自旋密度(MSD),临界点(CP)特征值(分子中的原子[AIM]分析),以及自然键轨道(NBO)分析,包括自然种群分析(NPA)和电荷转移能的变化(ΔE CT)。我们的结果表明,在Au中存在3 NC,所述N个的二苯醚氢,氧 H键在两个ON增加 ħ 的Au 3(1)和ON ö ħ 的Au 3(2(ON)与它们的游离形式相比 H和ON ö 分别H,)。换句话说,在金的存在下,使这些键解离并形成其相应的RNS(1'和2')的趋势降低。这对于将O所观察到的刚好相反 H键H中 ö ħ 的Au 3(3),H ö ö ħ 的Au 3(4)及其相应的ROS(3'和4')。因此,我们可以得出结论,与ROS相比,金在RNS面前表现出不同的行为,而ROS比ROS更好。
更新日期:2020-08-24
中文翻译:

处于自由基生成和清除的密度泛函理论水平的十字路口的黄金:面对纳米金,氮和氧自由基与它们的前体
在我们之前的报告(J. Phys。Org。Chem。,2017)中,我们讨论了金纳米团簇(Au 3 NC)的双重行为,该团簇清除了活性氧(ROS)并在较小程度上促进了它们的生成。继续这个任务,我们研究金的影响,3 NC上常见的活性氮类(RNS:ON˙和ON˙ O)及其前体(ON H和ON Ò H,分别在理论上达到B3LYP / LACVP + *水平。我们比较结果与那些流行的ROS(H 氧,氢 Ò O)及其前体(H Ò H和H ø Ò H,分别地)。为此,探索了各种参数,例如结合能(E b),键解离能(BDE),键长,穆利坎自旋密度(MSD),临界点(CP)特征值(分子中的原子[AIM]分析),以及自然键轨道(NBO)分析,包括自然种群分析(NPA)和电荷转移能的变化(ΔE CT)。我们的结果表明,在Au中存在3 NC,所述N个的二苯醚氢,氧 H键在两个ON增加 ħ 的Au 3(1)和ON ö ħ 的Au 3(2(ON)与它们的游离形式相比 H和ON ö 分别H,)。换句话说,在金的存在下,使这些键解离并形成其相应的RNS(1'和2')的趋势降低。这对于将O所观察到的刚好相反 H键H中 ö ħ 的Au 3(3),H ö ö ħ 的Au 3(4)及其相应的ROS(3'和4')。因此,我们可以得出结论,与ROS相比,金在RNS面前表现出不同的行为,而ROS比ROS更好。











































 京公网安备 11010802027423号
京公网安备 11010802027423号