当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational study on the Rh‐catalyzed C?C activation of cyclopropanol to construct diketone or monoketone
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2020-08-24 , DOI: 10.1002/qua.26438 Tao Liu 1, 2 , Ping Wang 2 , Hong Ren 3
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2020-08-24 , DOI: 10.1002/qua.26438 Tao Liu 1, 2 , Ping Wang 2 , Hong Ren 3
Affiliation
|
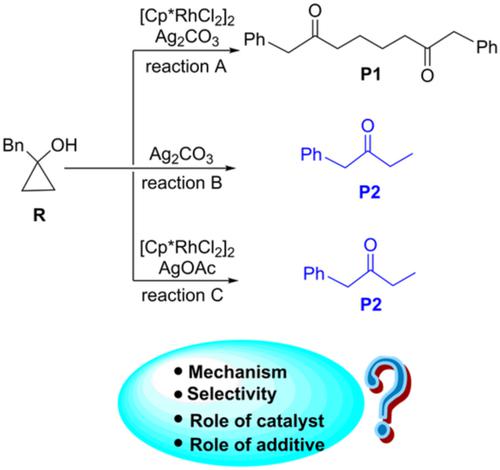
The mechanisms of CC activation of 1‐benzylcyclopropan‐1‐ol to produce 1,6‐diketone have been investigated by density functional theory calculations. The catalyst [Cp*RhCl2]2 and additive Ag2CO3 play an important role in controlling the selectivity. By using [Cp*RhCl2]2 as the catalyst and Ag2CO3 as the additive, the product is 1,6‐diketone, whereas the β‐hydride elimination product could not be obtained. The product would become monoketone in the absence of [Cp*RhCl2]2. In addition, the combination of catalyst [Cp*RhCl2]2 and additive AgOAc would also lead to monoketone. The observed selectivity could be attributed to the electronic effect.
中文翻译:

Rh催化的C ?的计算研究。C活化环丙醇以构建二酮或单酮
C的机制 C活化1- benzylcyclopropan -1-醇以产生1,6-己二酮的已经通过密度泛函理论计算的影响。催化剂[Cp * RhCl 2 ] 2和添加剂Ag 2 CO 3在控制选择性中起重要作用。通过使用[Cp * RhCl 2 ] 2作为催化剂和Ag 2 CO 3作为添加剂,产物为1,6-二酮,而无法获得β-氢化物消除产物。在没有[Cp * RhCl 2 ] 2的情况下,该产物将变成单酮。另外,催化剂[Cp * RhCl的组合2 ] 2和添加剂AgOAc也会产生单酮。观察到的选择性可以归因于电子效应。
更新日期:2020-08-24
中文翻译:

Rh催化的C ?的计算研究。C活化环丙醇以构建二酮或单酮
C的机制 C活化1- benzylcyclopropan -1-醇以产生1,6-己二酮的已经通过密度泛函理论计算的影响。催化剂[Cp * RhCl 2 ] 2和添加剂Ag 2 CO 3在控制选择性中起重要作用。通过使用[Cp * RhCl 2 ] 2作为催化剂和Ag 2 CO 3作为添加剂,产物为1,6-二酮,而无法获得β-氢化物消除产物。在没有[Cp * RhCl 2 ] 2的情况下,该产物将变成单酮。另外,催化剂[Cp * RhCl的组合2 ] 2和添加剂AgOAc也会产生单酮。观察到的选择性可以归因于电子效应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号