当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Redox-Neutral TEMPO Catalysis: Direct Radical (Hetero)Aryl C-H Di- and Trifluoromethoxylation.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-08-23 , DOI: 10.1002/anie.202009490 Johnny W Lee 1 , Sanghyun Lim 1 , Daniel N Maienshein 2 , Peng Liu 2 , Ming-Yu Ngai 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-08-23 , DOI: 10.1002/anie.202009490 Johnny W Lee 1 , Sanghyun Lim 1 , Daniel N Maienshein 2 , Peng Liu 2 , Ming-Yu Ngai 1
Affiliation
|
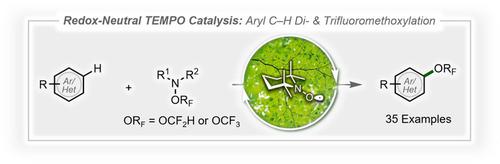
Applications of TEMPO. catalysis for the development of redox‐neutral transformations are rare. Reported here is the first TEMPO.‐catalyzed, redox‐neutral C−H di‐ and trifluoromethoxylation of (hetero)arenes. The reaction exhibits a broad substrate scope, has high functional‐group tolerance, and can be employed for the late‐stage functionalization of complex druglike molecules. Kinetic measurements, isolation and resubjection of catalytic intermediates, UV/Vis studies, and DFT calculations support the proposed oxidative TEMPO./TEMPO+ redox catalytic cycle. Mechanistic studies also suggest that Li2CO3 plays an important role in preventing catalyst deactivation. These findings will provide new insights into the design and development of novel reactions through redox‐neutral TEMPO. catalysis.
中文翻译:

氧化还原中性 TEMPO 催化:直接自由基(杂)芳基 CH 二氟甲氧基化和三氟甲氧基化。
TEMPO 的应用。氧化还原中性转化发展的催化作用很少见。这里报道的是第一个TEMPO 。(杂)芳烃的催化氧化还原中性 C−H 二氟甲氧基化和三氟甲氧基化。该反应表现出广泛的底物范围,具有较高的官能团耐受性,可用于复杂药物分子的后期功能化。动力学测量、催化中间体的分离和重新研究、UV/Vis 研究和 DFT 计算支持了所提出的氧化 TEMPO 。/TEMPO +氧化还原催化循环。机理研究还表明Li 2 CO 3在防止催化剂失活方面发挥着重要作用。这些发现将为通过氧化还原中性 TEMPO 设计和开发新型反应提供新的见解。催化。
更新日期:2020-08-23
中文翻译:

氧化还原中性 TEMPO 催化:直接自由基(杂)芳基 CH 二氟甲氧基化和三氟甲氧基化。
TEMPO 的应用。氧化还原中性转化发展的催化作用很少见。这里报道的是第一个TEMPO 。(杂)芳烃的催化氧化还原中性 C−H 二氟甲氧基化和三氟甲氧基化。该反应表现出广泛的底物范围,具有较高的官能团耐受性,可用于复杂药物分子的后期功能化。动力学测量、催化中间体的分离和重新研究、UV/Vis 研究和 DFT 计算支持了所提出的氧化 TEMPO 。/TEMPO +氧化还原催化循环。机理研究还表明Li 2 CO 3在防止催化剂失活方面发挥着重要作用。这些发现将为通过氧化还原中性 TEMPO 设计和开发新型反应提供新的见解。催化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号