Tetrahedron ( IF 2.1 ) Pub Date : 2020-08-24 , DOI: 10.1016/j.tet.2020.131478 Vakhid A. Mamedov , Vera L. Mamedova , Victor V. Syakaev , Julia K. Voronina , Essam M. Mahrous , Dmitry E. Korshin , Shamil K. Latypov , Oleg G. Sinyashin
|
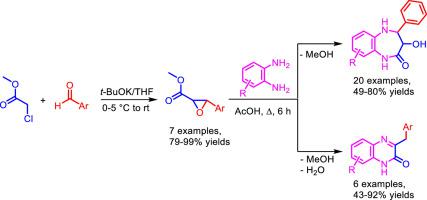
Representatives of two pharmacologically significant classes of compounds – 3-hydroxy-4-aryl-3,4,5-trihydro-2H-benzo[b][1,4]diazepin-2(1H)-ones and 3-benzylquinoxalin-2(1H)-ones – obtained in reactions of 1,2-diaminobenzenes with methyl 3-arylglycidates in boiling acetic acid. Substituents in arylglycidates determine the direction of processes. Electron withdrawing substituents (NO2), halogen atoms (Cl, Br, F), as well as the absence of substituents, provide the formation of benzo[b][1,4]diazepin-2(1H)-one derivatives, and electron donating groups (OMe, Me) contribute to the formation of 3-benzylquinoxalin-2(1H)-ones. As a result, a new rare representatives of 3-hydroxy-4-aryl-3,4,5-trihydro-2H-benzo[b][1,4]diazepin-2(1H)-ones were obtained and a new method for producing 3-benzylquinoxalin-2(1H)-ones has been proposed.
中文翻译:

3-羟基-4-芳基-3,4,5-三氢-2的区域选择性合成ħ -苯并[ b ] [1,4]二氮杂-2(1 ħ) -酮和3- benzylquinoxalin-2(1 ħ)暴露于1,2-二氨基苯时来自芳基缩水甘油酸酯的-
两类具有重要药理意义的化合物的代表– 3-羟基-4-芳基-3,4,5-三氢-2 H-苯并[ b ] [1,4]二氮杂-2-2 (1 H)-酮和3-苄基喹喔啉-2(1 H)-ones-由1,2-二氨基苯与3-芳基缩水甘油酸甲酯在沸腾的乙酸中反应制得。芳基缩水甘油酸酯中的取代基决定了过程的方向。吸电子取代基(NO2),卤素原子(Cl,Br,F)以及不存在取代基的情况下,形成了苯并[b] [1,4]二氮杂-2-2(1H)-一衍生物和电子捐赠基团(OMe,Me)有助于3-苄基喹喔啉-2(1H)-one的形成。结果,出现了3-羟基-4-芳基-3,4,5-三氢-2 H-苯并[[获得了b ] [1,4] diazepin-2(1 H)-one,并提出了一种新的制备3-苄基喹喔啉-2(1H)-one的方法。










































 京公网安备 11010802027423号
京公网安备 11010802027423号