Tetrahedron ( IF 2.1 ) Pub Date : 2020-08-23 , DOI: 10.1016/j.tet.2020.131474 Youcai Ma , Ruimei Xiong , Yangyang Feng , Xiaohui Zhang , Yan Xiong
|
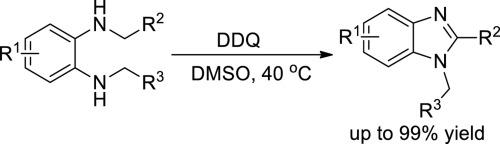
The synthetic methodology of 1,2-disubstituted benzimidazoles has been developed, which starts from N,N′-dialkyl o-phenylenediamines via intramolecular dehydrogenative coupling under the oxidation of DDQ with mild conditions. Through detailed optimization of reaction conditions, only DDQ was found essential without any additives to reach to the highest yield of 99%. In the cases of linear aliphatic substituents, the synthesis of 1-alkyl-2-phenylbenzimidazoles showed high selectivities and their structures were identified by 2D NMR COSY correlation analysis. A plausible mechanism was proposed to interpret the observed reactivities and selectivities.
中文翻译:

N,N'-二烷基邻苯二胺的DDQ氧化的分子内脱氢偶联反应合成1,2-二取代的苯并咪唑
已经开发了1,2-二取代的苯并咪唑的合成方法,该方法从N,N'-二烷基邻苯二胺开始,在温和的条件下,通过DDQ的分子内脱氢偶联作用。通过详细优化反应条件,发现只有DDQ才是必需的,而无需添加任何添加剂即可达到99%的最高收率。在直链脂族取代基的情况下,1-烷基-2-苯基苯并咪唑的合成显示出高选择性,并且通过2D NMR COZY相关分析鉴定了它们的结构。提出了一种合理的机制来解释观察到的反应性和选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号