Microporous and Mesoporous Materials ( IF 4.8 ) Pub Date : 2020-08-24 , DOI: 10.1016/j.micromeso.2020.110573 Ebenezer C. Nnadozie , Peter A. Ajibade
|
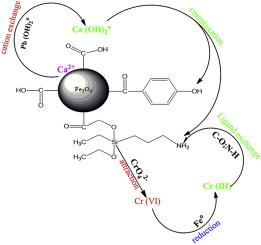
Magnetic composite (Fe3O4@BC/APTES) functionalized with 3-aminopropyl triethoxysilane (APTES) was prepared by capping magnetite nanoparticles with biochar prepared from the root of C. odorata and used as adsorbent for the adsorption of Pb(II) and Cr(VI) from aqueous solution. 93.95% of Pb(II) and 52.8% of Cr(VI) were successfully adsorbed from aqueous solution by the adsorbent after 1 h at 20 °C. The Langmuir equation best described the adsorption data with maximum adsorption capacities of 64.92 mg/g (R2 = 0.97) and 48.86 mg/g (R2 = 0.92) for Pb(II) and Cr(VI) respectively. The adsorption process was investigated using the intraparticle diffusion model together with the pseudo first and second order kinetic equations. The composite was stable in an alkaline environment while 8.55% of iron was leached in an acidic medium after 1 h and the results shows Pb(II) adsorption was not affected by competing ions. Pb(II) was better adsorbed at alkaline pH as against acidic pH for Cr(VI). 88% of adsorbed Cr(VI) ion was effectively desorbed using 0.005 M HNO3 after 4 cycles. Thermodynamic parameter suggested that the sorption process was by physisorption and exothermically moderated. The composite was magnetic and easily recovered with a small bar magnet from aqueous solution after 20 s.
中文翻译:

APTES功能化磁性生物炭对Pb(II)和Cr(VI)离子的吸附,动力学和机理研究
用3-氨基丙基三乙氧基硅烷(APTES)官能化的磁性复合物(Fe 3 O 4 @ BC / APTES)通过用香茅根部制备的生物炭覆盖磁铁矿纳米颗粒,并用作吸附Pb(II)和水溶液中的Cr(VI)。在20°C下放置1小时后,吸附剂成功地从水溶液中吸附了93.95%的Pb(II)和52.8%的Cr(VI)。Langmuir方程最能描述吸附数据,最大吸附容量为64.92 mg / g(R 2 = 0.97)和48.86 mg / g(R 2 分别为Pb(II)和Cr(VI)= 0.92)。使用粒子内扩散模型以及伪一级和二级动力学方程式研究了吸附过程。复合材料在碱性环境中稳定,而1h后8.55%的铁在酸性介质中浸出,结果表明Pb(II)的吸附不受竞争离子的影响。与Cr(VI)的酸性pH相比,Pb(II)在碱性pH下的吸附更好。4个循环后,使用0.005 M HNO 3有效地解吸了88%的吸附的Cr(VI)离子。热力学参数表明吸附过程是通过物理吸附并放热缓和的。该复合材料是磁性的,并且在20秒后可以用小条形磁铁轻松地从水溶液中回收。











































 京公网安备 11010802027423号
京公网安备 11010802027423号