Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inverse Electron Demand Diels-Alder Reactions in the Liposomal Membrane Accelerates Release of the Encapsulated Drugs.
Langmuir ( IF 3.7 ) Pub Date : 2020-08-22 , DOI: 10.1021/acs.langmuir.0c01525 Kento Kannaka 1 , Kohei Sano 1 , Hiromichi Nakahara 2 , Masayuki Munekane 1 , Masayori Hagimori 1, 3 , Toshihide Yamasaki 1 , Takahiro Mukai 1
Langmuir ( IF 3.7 ) Pub Date : 2020-08-22 , DOI: 10.1021/acs.langmuir.0c01525 Kento Kannaka 1 , Kohei Sano 1 , Hiromichi Nakahara 2 , Masayuki Munekane 1 , Masayori Hagimori 1, 3 , Toshihide Yamasaki 1 , Takahiro Mukai 1
Affiliation
|
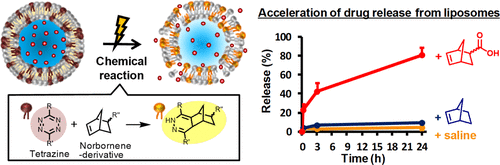
Bio-orthogonal inverse electron demand Diels–Alder (IEDDA) reactions between liposomes containing a tetrazine-based (Tz) compound and 2-norbornene (2-NB) could be a novel trigger for accelerating drug release from the liposomes via temporary membrane destabilization, as shown in our previous report. Herein, we evaluated the in vitro drug release using NB derivatives with carboxyl groups [5-norbornene-2-carboxylic acid (NBCOOH) and 5-norbornene-2,3-dicarboxylic acid (NB(COOH)2)] to investigate the effects of substituents at the NB backbone on the drug release rate. First, POTz-liposome composed of a Tz compound (2-hexadecyl-N-(6-(6-(pyridin-2-yl)-1,2,4,5-tetrazin-3-yl)pyridin-3-yl)octadecanamide) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine (POPC) were prepared. The mass spectrometry analysis revealed the binding of NB derivatives to the Tz compound via the IEDDA reaction after the POTz-liposome reacted with the NB derivatives. Indium-111-labeled diethylenetriaminepentaacetic acid (111In-DTPA) was encapsulated inside the liposomes, and the drug release rate was quantified by measuring radioactivity. At 24 h after incubation with 2-NB, NBCOOH, and NB(COOH)2, the release rates of 111In-DTPA from POTz-liposome were 21.0, 80.8, and 23.3%, respectively, which were significantly higher than those of POTz-liposome that was not treated with NB derivatives (4.2%), indicating the involvement of the IEDDA reaction for prompting drug release. Additionally, a thermodynamic evaluation using Langmuir monolayers was conducted to explore the mechanism of the accelerated drug release. An increase in membrane fluidity and a reduction in intermolecular repulsion between POPC and the Tz compound were observed after the reaction with NB derivatives, especially for NBCOOH. Thus, the IEDDA reaction in the liposomal membrane could be a potent trigger for accelerating the release of encapsulated drugs by regulating membrane fluidity and intermolecular repulsion in the liposomal membrane.
中文翻译:

脂质体膜中的逆电子需求Diels-Alder反应加速了封装药物的释放。
包含四嗪基(Tz)化合物的脂质体与2-降冰片烯(2-NB)之间的生物正交逆电子需求Diels-Alder(IEDDA)反应可能是通过暂时的膜失稳来加速药物从脂质体释放的新触发因素,如我们先前的报告所示。在这里,我们评估了带有羧基的NB衍生物[5-降冰片烯-2-羧酸(NBCOOH)和5-降冰片烯-2,3-二羧酸(NB(COOH)2)]的体外药物释放以研究其作用。 NB骨架上的取代基对药物释放速率的影响。首先,由Tz化合物(2-十六烷基-N-(6-(6-(吡啶-2-基)-1,2,4,5-四嗪-3-基)吡啶-3-基)十八烷酰胺)和1-棕榈酰基-2-油酰基-制备了sn-甘油-3-磷脂酰胆碱(POPC)。质谱分析显示,在POTz-脂质体与NB衍生物反应后,NB衍生物通过IEDDA反应与Tz化合物结合。将铟111标记的二亚乙基三胺五乙酸(111 In-DTPA)封装在脂质体内,并通过测量放射性来定量药物释放速率。与2-NB,NBCOOH和NB(COOH)2孵育24小时后,释放速率为111来自POTz-脂质体的In-DTPA分别为21.0%,80.8和23.3%,显着高于未经NB衍生物处理的POTz-脂质体(4.2%),表明IEDDA反应参与了促药物释放。此外,进行了使用Langmuir单层的热力学评估,以探索加速药物释放的机制。与NB衍生物(尤其是NBCOOH)反应后,观察到膜流动性的增加和POPC与Tz化合物之间的分子间排斥的减少。因此,脂质体膜中的IEDDA反应可能是通过调节脂质体膜中的膜流动性和分子间排斥来加速包封药物释放的有效触发器。
更新日期:2020-09-15
中文翻译:

脂质体膜中的逆电子需求Diels-Alder反应加速了封装药物的释放。
包含四嗪基(Tz)化合物的脂质体与2-降冰片烯(2-NB)之间的生物正交逆电子需求Diels-Alder(IEDDA)反应可能是通过暂时的膜失稳来加速药物从脂质体释放的新触发因素,如我们先前的报告所示。在这里,我们评估了带有羧基的NB衍生物[5-降冰片烯-2-羧酸(NBCOOH)和5-降冰片烯-2,3-二羧酸(NB(COOH)2)]的体外药物释放以研究其作用。 NB骨架上的取代基对药物释放速率的影响。首先,由Tz化合物(2-十六烷基-N-(6-(6-(吡啶-2-基)-1,2,4,5-四嗪-3-基)吡啶-3-基)十八烷酰胺)和1-棕榈酰基-2-油酰基-制备了sn-甘油-3-磷脂酰胆碱(POPC)。质谱分析显示,在POTz-脂质体与NB衍生物反应后,NB衍生物通过IEDDA反应与Tz化合物结合。将铟111标记的二亚乙基三胺五乙酸(111 In-DTPA)封装在脂质体内,并通过测量放射性来定量药物释放速率。与2-NB,NBCOOH和NB(COOH)2孵育24小时后,释放速率为111来自POTz-脂质体的In-DTPA分别为21.0%,80.8和23.3%,显着高于未经NB衍生物处理的POTz-脂质体(4.2%),表明IEDDA反应参与了促药物释放。此外,进行了使用Langmuir单层的热力学评估,以探索加速药物释放的机制。与NB衍生物(尤其是NBCOOH)反应后,观察到膜流动性的增加和POPC与Tz化合物之间的分子间排斥的减少。因此,脂质体膜中的IEDDA反应可能是通过调节脂质体膜中的膜流动性和分子间排斥来加速包封药物释放的有效触发器。









































 京公网安备 11010802027423号
京公网安备 11010802027423号