当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photoinduced charge accumulation and prolonged multielectron storage for the separation of light and dark reaction
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-08-22 , DOI: 10.1021/jacs.0c03779 Martin Schulz 1, 2 , Nina Hagmeyer 1 , Frerk Wehmeyer 1 , Grace Lowe 3 , Marco Rosenkranz 4 , Bianca Seidler 1 , Alexey Popov 4 , Carsten Streb 3 , Johannes G Vos 5 , Benjamin Dietzek 1, 2, 6
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-08-22 , DOI: 10.1021/jacs.0c03779 Martin Schulz 1, 2 , Nina Hagmeyer 1 , Frerk Wehmeyer 1 , Grace Lowe 3 , Marco Rosenkranz 4 , Bianca Seidler 1 , Alexey Popov 4 , Carsten Streb 3 , Johannes G Vos 5 , Benjamin Dietzek 1, 2, 6
Affiliation
|
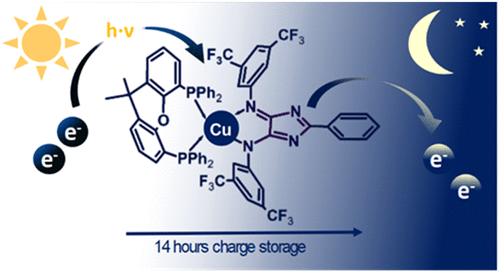
The utilization of solar energy is restricted by the intermittent nature of solar influx. We present novel noble-metal free complexes that can be photochemically charged in the presence of sacrificial electron donors and remain stable in its charged form for over 14 hours. This allows the doubly reduced Cu(I) 4H-imidazolate complex to be stored after photo-chemical charging and used as a reagent in dark reactions, such as the reduction of methyl viologen, or oxygen. Combined UV-Vis/EPR spectroelectrochemistry indicates that a two-electron reduction is induced by introducing sacrificial electron donors that facilitate proton-coupled electron transfer. Repeated photochemical reduction and chemical oxida-tion reveals that the complex retained a charging capacity of 72% after four cycles. We demonstrate a chemical system that can decouple photochemical processes from the day-night cycle, which has been a barrier to realizing utilization of solar energy in photochemical processes on a global scale.
中文翻译:

用于分离明暗反应的光致电荷积累和延长的多电子存储
太阳能的利用受到太阳涌入的间歇性的限制。我们提出了新型的无贵金属配合物,它们可以在牺牲电子供体的存在下进行光化学充电,并在其带电形式下保持稳定超过 14 小时。这使得双还原的 Cu(I) 4H-咪唑络合物在光化学充电后储存起来,并用作暗反应中的试剂,例如甲基紫精或氧气的还原。组合 UV-Vis/EPR 光谱电化学表明,通过引入促进质子耦合电子转移的牺牲电子供体,诱导了双电子还原。重复的光化学还原和化学氧化表明,该复合物在四次循环后仍保持 72% 的充电容量。
更新日期:2020-08-22
中文翻译:

用于分离明暗反应的光致电荷积累和延长的多电子存储
太阳能的利用受到太阳涌入的间歇性的限制。我们提出了新型的无贵金属配合物,它们可以在牺牲电子供体的存在下进行光化学充电,并在其带电形式下保持稳定超过 14 小时。这使得双还原的 Cu(I) 4H-咪唑络合物在光化学充电后储存起来,并用作暗反应中的试剂,例如甲基紫精或氧气的还原。组合 UV-Vis/EPR 光谱电化学表明,通过引入促进质子耦合电子转移的牺牲电子供体,诱导了双电子还原。重复的光化学还原和化学氧化表明,该复合物在四次循环后仍保持 72% 的充电容量。









































 京公网安备 11010802027423号
京公网安备 11010802027423号