当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Site-Specific Bioconjugation through Enzyme-Catalyzed Tyrosine–Cysteine Bond Formation
ACS Central Science ( IF 12.7 ) Pub Date : 2020-08-21 , DOI: 10.1021/acscentsci.0c00940 Marco J. Lobba 1 , Christof Fellmann 2, 3, 4 , Alan M. Marmelstein 1 , Johnathan C. Maza 1 , Elijah N. Kissman 1 , Stephanie A. Robinson 1 , Brett T. Staahl 2 , Cole Urnes 2 , Rachel J. Lew 3 , Casey S. Mogilevsky 1 , Jennifer A. Doudna 1, 2, 3, 5, 6 , Matthew B. Francis 1, 7
ACS Central Science ( IF 12.7 ) Pub Date : 2020-08-21 , DOI: 10.1021/acscentsci.0c00940 Marco J. Lobba 1 , Christof Fellmann 2, 3, 4 , Alan M. Marmelstein 1 , Johnathan C. Maza 1 , Elijah N. Kissman 1 , Stephanie A. Robinson 1 , Brett T. Staahl 2 , Cole Urnes 2 , Rachel J. Lew 3 , Casey S. Mogilevsky 1 , Jennifer A. Doudna 1, 2, 3, 5, 6 , Matthew B. Francis 1, 7
Affiliation
|
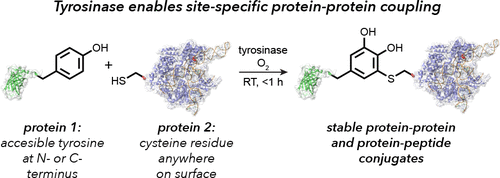
The synthesis of protein–protein and protein–peptide conjugates is an important capability for producing vaccines, immunotherapeutics, and targeted delivery agents. Herein we show that the enzyme tyrosinase is capable of oxidizing exposed tyrosine residues into o-quinones that react rapidly with cysteine residues on target proteins. This coupling reaction occurs under mild aerobic conditions and has the rare ability to join full-size proteins in under 2 h. The utility of the approach is demonstrated for the attachment of cationic peptides to enhance the cellular delivery of CRISPR-Cas9 20-fold and for the coupling of reporter proteins to a cancer-targeting antibody fragment without loss of its cell-specific binding ability. The broad applicability of this technique provides a new building block approach for the synthesis of protein chimeras.
中文翻译:

通过酶催化酪氨酸-半胱氨酸键形成的位点特异性生物缀合
蛋白质-蛋白质和蛋白质-肽结合物的合成是生产疫苗,免疫疗法和靶向递送剂的重要能力。在这里,我们表明酪氨酸酶能够将暴露的酪氨酸残基氧化为o-醌与目标蛋白上的半胱氨酸残基快速反应。这种偶联反应在温和的有氧条件下发生,并具有在2小时内加入全尺寸蛋白质的罕见能力。已证明该方法的实用性用于连接阳离子肽以增强CRISPR-Cas9的细胞递送20倍,以及将报告蛋白与靶向癌症的抗体片段偶联而不丧失其细胞特异性结合能力。该技术的广泛适用性为蛋白质嵌合体的合成提供了一种新的构建方法。
更新日期:2020-09-23
中文翻译:

通过酶催化酪氨酸-半胱氨酸键形成的位点特异性生物缀合
蛋白质-蛋白质和蛋白质-肽结合物的合成是生产疫苗,免疫疗法和靶向递送剂的重要能力。在这里,我们表明酪氨酸酶能够将暴露的酪氨酸残基氧化为o-醌与目标蛋白上的半胱氨酸残基快速反应。这种偶联反应在温和的有氧条件下发生,并具有在2小时内加入全尺寸蛋白质的罕见能力。已证明该方法的实用性用于连接阳离子肽以增强CRISPR-Cas9的细胞递送20倍,以及将报告蛋白与靶向癌症的抗体片段偶联而不丧失其细胞特异性结合能力。该技术的广泛适用性为蛋白质嵌合体的合成提供了一种新的构建方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号