European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-08-22 , DOI: 10.1016/j.ejmech.2020.112699 Diego G Ghiano 1 , Alejandro Recio-Balsells 1 , Ana Bortolotti 2 , Lucas A Defelipe 3 , Adrián Turjanski 3 , Héctor R Morbidoni 4 , Guillermo R Labadie 5
|
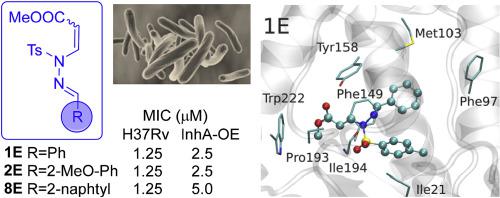
A library of thirty N-substituted tosyl N’-acryl-hydrazones was prepared with p-toluenesulfonyl hydrazide, methyl propiolate and different aldehydes in a one-pot synthesis via an aza-Michael reaction. The scope of the reaction was studied, including aliphatic, isoprenylic, aromatic and carbocyclic aldehydes. The prepared collection was tested against Mycobacterium tuberculosis H37Rv. Nine analogs of the collection showed Minimum Inhibitory Concentration ≤10 μM, of which the most active members (MIC of 1.25 μM) were exclusively E isomers. In order to validate the mechanism of action of the most active acrylates, we tested their activity on a M. tuberculosis InhA over-expressing strain obtaining MIC that consistently doubled those obtained on the wild type strain.
Additionally, the binding mode of those analogs on M. tuberculosis InhA was investigated by docking simulations. The results displayed a hydrogen bond interaction between the sulfonamide and Ile194 and the carbonyl of the methyl ester with Tyr 158 (both critical residues in the interaction with the fatty acyl chain substrate), where the main differences on the binding mode relays on the hydrophobicity of the nitrogen substituent. Additionally, chemoinformatic analysis was performed to evaluate in silico possible cytotoxicity risk and ADME-Tox profile.
Based on their simple preparation and interesting antimycobacterial activity profile, the newly prepared aza-acrylates are promising candidates for antitubercular drug development.
中文翻译:

异烟肼激发了新的一锅抗结核化合物的合成方法。
通过氮杂-迈克尔反应一锅合成,用对甲苯磺酰肼,丙酸甲酯和不同的醛制备了三十个N-取代的甲苯磺酰基N'-丙烯酸-hydr的文库。研究了反应范围,包括脂族,异戊烯基,芳族和碳环醛。测试所制备的收集物针对结核分枝杆菌H37Rv。该集合的九个类似物显示出最小抑菌浓度≤10μM,其中最活跃的成员(MIC为1.25μM)仅是E异构体。为了验证最具活性的丙烯酸酯的作用机理,我们测试了它们对结核分枝杆菌的活性 InhA过表达菌株获得的MIC始终是野生型菌株获得的MIC的两倍。
另外,通过对接模拟研究了那些类似物在结核分枝杆菌InhA上的结合模式。结果显示了磺酰胺和Ile194与甲基酯与Tyr 158之间的氢键相互作用(两个关键残基均与脂肪酰基链底物相互作用),其中键合方式的主要差异取决于疏水性。氮取代基。另外,进行了化学信息学分析以评估计算机可能的细胞毒性风险和ADME-Tox谱。
基于它们的简单制备和有趣的抗分枝杆菌活性特征,新制备的氮杂丙烯酸酯是抗结核药物开发的有希望的候选者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号