当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Factors Responsible for the Aggregation of Poly(vinyl alcohol) in Aqueous Solution as Revealed by Molecular Dynamics Simulations
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-08-21 , DOI: 10.1021/acs.iecr.0c02467 Raviteja Kurapati 1 , Upendra Natarajan 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-08-21 , DOI: 10.1021/acs.iecr.0c02467 Raviteja Kurapati 1 , Upendra Natarajan 1
Affiliation
|
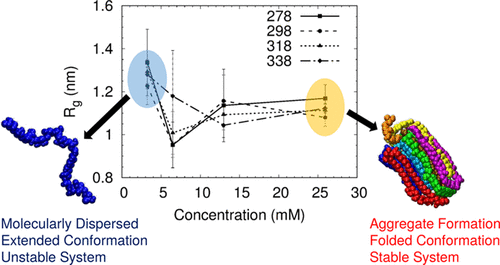
Molecular dynamics simulations are used to study the structure and dynamics of poly(vinyl alcohol) and water in aqueous solution as a function of concentration at different temperatures in the range of 278–338 K. Simulations were performed using multiple oligomeric chains for facilitating interchain interactions as well as a direct comparison with experimental data. PVA chains fold and bundle up to form an aggregate in solution. The intermolecular spatial distributions show the structure of aggregate to be ordered. PVA chains show a high tendency to form intrachain hydrogen bonds between adjacent repeating units, instead of interchain H-bonds, indicating hydrophobic effect as the major driving force for aggregate formation. At all temperatures, the conformations of a single PVA chain by itself in solution are unstable, going back and forth between extended and folded states. However, interchain interactions among PVA chains in the aggregate stabilize the folded conformation. An increase in temperature results in faster motions and an increase in concentration results in slower dynamics. At higher concentration, the chains adopt a single folded state independent of temperature so that there is an insignificant effect on Rg. The competition between the formation of various hydrogen bonds such as intrachain, interchain, and PVA–water is the key to understand the solvation behavior of PVA. The activation energy for the conformational transition between the trans and gauche states of backbone dihedrals obtained from the simulations is 15.73 kJ/mol, which is close to the value of 13.4 kJ/mol obtained from experiments for 15 wt % PVA solution. The hydrophobic effect rather than interchain PVA hydrogen bonding is the major driving force for the aggregation of PVA in water.
中文翻译:

分子动力学模拟揭示了聚乙烯醇在水溶液中聚集的影响因素
分子动力学模拟用于研究水溶液中聚乙烯醇和水的结构和动力学,以及在278–338 K范围内不同温度下浓度的函数。使用多个低聚物链进行模拟以促进链间相互作用以及与实验数据的直接比较。PVA链折叠并捆绑在一起,形成溶液中的聚集体。分子间的空间分布显示了有序聚集体的结构。PVA链显示出在相邻重复单元之间形成链内氢键的趋势,而不是链间H键,这表明疏水作用是聚集体形成的主要驱动力。在所有温度下,溶液中单个PVA链的构象都是不稳定的,在扩展状态和折叠状态之间来回移动。然而,聚集体中PVA链之间的链间相互作用稳定了折叠构象。温度升高导致运动更快,而浓度升高则导致运动变慢。浓度较高时,链条呈单折叠状态,不受温度影响,因此对链条的影响很小ř克。各种氢键(如链内,链间和PVA-水)的形成之间的竞争是了解PVA溶剂化行为的关键。通过模拟获得的骨架二面体的反式和gauche状态之间构象转变的活化能为15.73 kJ / mol,接近于15 wt%PVA溶液的实验获得的13.4 kJ / mol的值。疏水作用而不是链间PVA氢键是水中PVA聚集的主要驱动力。
更新日期:2020-09-16
中文翻译:

分子动力学模拟揭示了聚乙烯醇在水溶液中聚集的影响因素
分子动力学模拟用于研究水溶液中聚乙烯醇和水的结构和动力学,以及在278–338 K范围内不同温度下浓度的函数。使用多个低聚物链进行模拟以促进链间相互作用以及与实验数据的直接比较。PVA链折叠并捆绑在一起,形成溶液中的聚集体。分子间的空间分布显示了有序聚集体的结构。PVA链显示出在相邻重复单元之间形成链内氢键的趋势,而不是链间H键,这表明疏水作用是聚集体形成的主要驱动力。在所有温度下,溶液中单个PVA链的构象都是不稳定的,在扩展状态和折叠状态之间来回移动。然而,聚集体中PVA链之间的链间相互作用稳定了折叠构象。温度升高导致运动更快,而浓度升高则导致运动变慢。浓度较高时,链条呈单折叠状态,不受温度影响,因此对链条的影响很小ř克。各种氢键(如链内,链间和PVA-水)的形成之间的竞争是了解PVA溶剂化行为的关键。通过模拟获得的骨架二面体的反式和gauche状态之间构象转变的活化能为15.73 kJ / mol,接近于15 wt%PVA溶液的实验获得的13.4 kJ / mol的值。疏水作用而不是链间PVA氢键是水中PVA聚集的主要驱动力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号