当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protein Splicing Activity of the Haloferax volcanii PolB-c Intein Is Sensitive to Homing Endonuclease Domain Mutations.
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-21 , DOI: 10.1021/acs.biochem.0c00512 Shachar Robinzon 1 , Alexandra R Cawood 2 , Mercedes A Ruiz 2 , Uri Gophna 1 , Neta Altman-Price 1, 3 , Kenneth V Mills 2
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-21 , DOI: 10.1021/acs.biochem.0c00512 Shachar Robinzon 1 , Alexandra R Cawood 2 , Mercedes A Ruiz 2 , Uri Gophna 1 , Neta Altman-Price 1, 3 , Kenneth V Mills 2
Affiliation
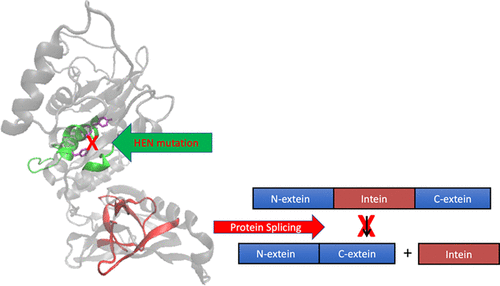
|
Inteins are selfish genetic elements residing in open reading frames that can splice post-translationally, resulting in the ligation of an uninterrupted, functional protein. Like other inteins, the DNA polymerase B (PolB) intein of the halophilic archaeon Haloferax volcanii has an active homing endonuclease (HEN) domain, facilitating its horizontal transmission. Previous work has shown that the presence of the PolB intein exerts a significant fitness cost on the organism compared to an intein-free isogenic H. volcanii. Here, we show that mutation of a conserved residue in the HEN domain not only reduces intein homing but also slows growth. Surprisingly, although this mutation is far from the protein splicing active site, it also significantly reduces in vitro protein splicing. Moreover, two additional HEN domain mutations, which could not be introduced to H. volcanii, presumably due to lethality, also eliminate protein splicing activity in vitro. These results suggest an interplay between HEN residues and the protein splicing domain, despite an over 35 Å separation in a PolB intein homology model. The combination of in vivo and in vitro evidence strongly supports a model of codependence between the self-splicing domain and the HEN domain that has been alluded to by previous in vitro studies of protein splicing with HEN domain-containing inteins.
中文翻译:

Haloferax volcanii PolB-c Intein 的蛋白质剪接活性对归巢核酸内切酶域突变敏感。
内含肽是位于开放阅读框中的自私遗传元件,可以在翻译后进行剪接,从而导致不间断的功能性蛋白质的连接。与其他内含肽一样,嗜盐古菌Haloferax volcanii的 DNA 聚合酶 B (PolB) 内含肽具有活性归巢核酸内切酶 (HEN) 域,促进其水平传输。以前的工作已经表明,POLB的存在内含肽施加相比,无内含肽等基因在生物体中的显著健身成本H. volcanii。在这里,我们表明 HEN 域中保守残基的突变不仅会减少内含肽的归巢,还会减缓生长。令人惊讶的是,虽然这种突变远离蛋白质剪接活性位点,但在体外也显着降低蛋白质剪接。此外,两个额外的 HEN 域突变,可能是由于致死性而无法引入H. volcanii,也消除了体外蛋白质剪接活性。这些结果表明 HEN 残基和蛋白质剪接结构域之间存在相互作用,尽管在 PolB 内含肽同源模型中分离超过 35 埃。的组合在体内和体外证据强烈支持自我剪接结构域和已提到通过先前所述HEN域之间相互依赖的模型体外与HEN结构域的内含肽蛋白剪接的研究。
更新日期:2020-09-15
中文翻译:

Haloferax volcanii PolB-c Intein 的蛋白质剪接活性对归巢核酸内切酶域突变敏感。
内含肽是位于开放阅读框中的自私遗传元件,可以在翻译后进行剪接,从而导致不间断的功能性蛋白质的连接。与其他内含肽一样,嗜盐古菌Haloferax volcanii的 DNA 聚合酶 B (PolB) 内含肽具有活性归巢核酸内切酶 (HEN) 域,促进其水平传输。以前的工作已经表明,POLB的存在内含肽施加相比,无内含肽等基因在生物体中的显著健身成本H. volcanii。在这里,我们表明 HEN 域中保守残基的突变不仅会减少内含肽的归巢,还会减缓生长。令人惊讶的是,虽然这种突变远离蛋白质剪接活性位点,但在体外也显着降低蛋白质剪接。此外,两个额外的 HEN 域突变,可能是由于致死性而无法引入H. volcanii,也消除了体外蛋白质剪接活性。这些结果表明 HEN 残基和蛋白质剪接结构域之间存在相互作用,尽管在 PolB 内含肽同源模型中分离超过 35 埃。的组合在体内和体外证据强烈支持自我剪接结构域和已提到通过先前所述HEN域之间相互依赖的模型体外与HEN结构域的内含肽蛋白剪接的研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号