当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Downhill (Un)Folding Coupled to Binding as a Mechanism for Engineering Broadband Protein Conformational Transducers.
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-08-21 , DOI: 10.1021/acssynbio.0c00190 Suhani Nagpal 1, 2 , Thinh D N Luong 2, 3 , Mourad Sadqi 2, 4 , Victor Muñoz 1, 2, 3, 4
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-08-21 , DOI: 10.1021/acssynbio.0c00190 Suhani Nagpal 1, 2 , Thinh D N Luong 2, 3 , Mourad Sadqi 2, 4 , Victor Muñoz 1, 2, 3, 4
Affiliation
|
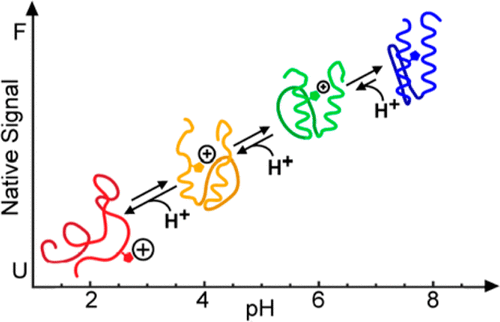
Canonical proteins fold and function as conformational switches that toggle between their folded (on) and unfolded (off) states, a mechanism that also provides the basis for engineering transducers for biosensor applications. One of the limitations of such transducers, however, is their relatively narrow operational range, limited to ligand concentrations 20-fold below or above their C50. Previously, we discovered that certain fast-folding proteins lose/gain structure gradually (downhill folding), which led us to postulate their operation as conformational rheostats capable of processing inputs/outputs in analog fashion. Conformational rheostats could make transducers with extended sensitivity. Here we investigate this hypothesis by engineering pH transducing into the naturally pH insensitive, downhill folding protein gpW. Particularly, we engineered histidine grafts into its hydrophobic core to induce unfolding via histidine ionization. We designed and tested the effects of ionization via computational modeling and studied experimentally the four most promising single grafts and two double grafts. All tested mutants become reversible pH transducers in the 4–9 range, and their response increases proportionally to how buried the histidine graft is. Importantly, the pH-dependent reversible (un)folding occurs in rheostatic fashion, so the engineered transducers can detect up to 6 orders of magnitude in [H+] for single grafts, and even more for double grafts. Our results demonstrate that downhill (un)folding coupled to binding produces the gradual, analog responses to the ligand (here H+) that are expected of conformational rheostats, and which make them a powerful mechanism for engineering transducers with sensitivity over many orders of magnitude in ligand concentration (broadband).
中文翻译:

下坡(非)折叠与结合结合作为工程宽带蛋白质构象传感器的机制。
经典蛋白质折叠并充当构象开关,在折叠(开启)和展开(关闭)状态之间切换,这种机制也为生物传感器应用的工程传感器提供了基础。然而,此类传感器的局限性之一是其操作范围相对较窄,仅限于配体浓度低于或高于其 C 50 20 倍. 以前,我们发现某些快速折叠的蛋白质逐渐失去/获得结构(下坡折叠),这使我们假设它们的操作是能够以模拟方式处理输入/输出的构象变阻器。构象变阻器可以使传感器具有扩展的灵敏度。在这里,我们通过将 pH 值转化为天然 pH 不敏感的下坡折叠蛋白 gpW 来研究这一假设。特别是,我们将组氨酸移植物工程化到其疏水核心中,以通过组氨酸电离诱导解折叠。我们设计并测试了电离的效果,通过计算建模并通过实验研究了四种最有希望的单移植物和两种双移植物。所有测试的突变体都成为 4-9 范围内的可逆 pH 传感器,它们的响应与组氨酸移植物的掩埋程度成正比。重要的是,依赖于 pH 的可逆(未)折叠以变阻方式发生,因此工程传感器可以检测到单移植物的[H + ]高达 6 个数量级,对于双移植物甚至更多。我们的结果表明,与结合偶联的下坡(非)折叠会产生对配体(此处为 H +) 是构象变阻器所期望的,这使它们成为工程传感器的强大机制,其灵敏度超过许多数量级的配体浓度(宽带)。
更新日期:2020-09-20
中文翻译:

下坡(非)折叠与结合结合作为工程宽带蛋白质构象传感器的机制。
经典蛋白质折叠并充当构象开关,在折叠(开启)和展开(关闭)状态之间切换,这种机制也为生物传感器应用的工程传感器提供了基础。然而,此类传感器的局限性之一是其操作范围相对较窄,仅限于配体浓度低于或高于其 C 50 20 倍. 以前,我们发现某些快速折叠的蛋白质逐渐失去/获得结构(下坡折叠),这使我们假设它们的操作是能够以模拟方式处理输入/输出的构象变阻器。构象变阻器可以使传感器具有扩展的灵敏度。在这里,我们通过将 pH 值转化为天然 pH 不敏感的下坡折叠蛋白 gpW 来研究这一假设。特别是,我们将组氨酸移植物工程化到其疏水核心中,以通过组氨酸电离诱导解折叠。我们设计并测试了电离的效果,通过计算建模并通过实验研究了四种最有希望的单移植物和两种双移植物。所有测试的突变体都成为 4-9 范围内的可逆 pH 传感器,它们的响应与组氨酸移植物的掩埋程度成正比。重要的是,依赖于 pH 的可逆(未)折叠以变阻方式发生,因此工程传感器可以检测到单移植物的[H + ]高达 6 个数量级,对于双移植物甚至更多。我们的结果表明,与结合偶联的下坡(非)折叠会产生对配体(此处为 H +) 是构象变阻器所期望的,这使它们成为工程传感器的强大机制,其灵敏度超过许多数量级的配体浓度(宽带)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号