当前位置:
X-MOL 学术
›
J. Polym. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into the polymerization kinetics of thermoresponsive polytrimethylene carbonate bearing a methoxyethoxy side group
Journal of Polymer Science ( IF 3.9 ) Pub Date : 2020-08-21 , DOI: 10.1002/pol.20200371 Amanda J. Brissenden 1 , Brian G. Amsden 1
Journal of Polymer Science ( IF 3.9 ) Pub Date : 2020-08-21 , DOI: 10.1002/pol.20200371 Amanda J. Brissenden 1 , Brian G. Amsden 1
Affiliation
|
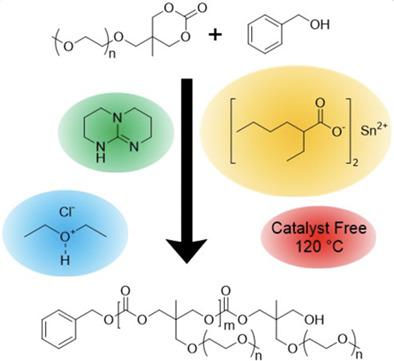
The ring‐opening polymerization kinetics of 5‐[2‐(2‐methoxyethoxy)‐ethoxymethyl]‐5‐methyl‐1,3‐dioxa‐2‐one (TMOE‐2) and 5‐[2‐{2‐(2‐methoxyethoxy)ethyoxy}‐ethoxymethyl]‐5‐methyl‐1,3‐dioxa‐2‐one (TMOE‐3) was investigated using different catalysts with the aim to improve control over molecular weight. The possibility of monomer impurities driving the variability in molecular weight that has been seen in different reports, was assessed and evidence of catalysis via an imidazole impurity was found. The catalysts 1,5,7‐triazobicyclo(4.4.0)dec‐5‐ene (TBD), hydrogen chloride in diethyl ether (HCl·Et2O), stannous 2‐ethylhexanoate (SnOct2), and catalyst free thermal polymerizations were conducted to understand the mechanisms influencing the molecular weight. TBD and HCl·Et2O consistently achieved high conversion of the monomer; however, molecular weights greater than 7,000 Da could not be achieved due to competing side reactions. SnOct2 catalyzed and catalyst free thermal polymerizations were highly influenced by monomer purity and achieved lower conversion than TBD and HCl·Et2O. Understanding these mechanisms will guide future synthesis of poly(TMOE‐2) and poly(TMOE‐3) for biomedical applications.
中文翻译:

对带有甲氧基乙氧基侧基的热敏性聚三亚甲基碳酸酯的聚合动力学的见解
5- [2-(2-甲氧基乙氧基)-乙氧基甲基] -5-甲基-1,3-二氧杂-2-酮(TMOE-2)和5 [2--2-(2)的开环聚合动力学为了改善对分子量的控制,使用了不同的催化剂,对[甲氧基乙氧基]乙氧基}乙氧基甲基] -5-甲基-1,3-二氧杂-2-酮(TMOE-3)进行了研究。评估了在不同报告中发现的单体杂质驱动分子量变化的可能性,并发现了通过咪唑杂质催化的证据。催化剂1,5,7-三偶氮二环(4.4.0)dec-5-烯(TBD),乙醚中的氯化氢(HCl·Et 2 O),2-乙基己酸亚锡(SnOct 2)和无催化剂的热聚合进行了了解影响分子量的机制。待定和HCl·Et 2O始终达到单体的高转化率;然而,由于竞争性的副反应,无法获得大于7,000 Da的分子量。SnOct 2催化的和不含催化剂的热聚合反应高度单体纯度的影响和比TBD和HCl的Et·实现较低的转化率2 O.了解这些机制将指导聚未来合成(TMOE-2)和聚(TMOE-3)用于生物医学应用程序。
更新日期:2020-10-02
中文翻译:

对带有甲氧基乙氧基侧基的热敏性聚三亚甲基碳酸酯的聚合动力学的见解
5- [2-(2-甲氧基乙氧基)-乙氧基甲基] -5-甲基-1,3-二氧杂-2-酮(TMOE-2)和5 [2--2-(2)的开环聚合动力学为了改善对分子量的控制,使用了不同的催化剂,对[甲氧基乙氧基]乙氧基}乙氧基甲基] -5-甲基-1,3-二氧杂-2-酮(TMOE-3)进行了研究。评估了在不同报告中发现的单体杂质驱动分子量变化的可能性,并发现了通过咪唑杂质催化的证据。催化剂1,5,7-三偶氮二环(4.4.0)dec-5-烯(TBD),乙醚中的氯化氢(HCl·Et 2 O),2-乙基己酸亚锡(SnOct 2)和无催化剂的热聚合进行了了解影响分子量的机制。待定和HCl·Et 2O始终达到单体的高转化率;然而,由于竞争性的副反应,无法获得大于7,000 Da的分子量。SnOct 2催化的和不含催化剂的热聚合反应高度单体纯度的影响和比TBD和HCl的Et·实现较低的转化率2 O.了解这些机制将指导聚未来合成(TMOE-2)和聚(TMOE-3)用于生物医学应用程序。











































 京公网安备 11010802027423号
京公网安备 11010802027423号