Reactive & Functional Polymers ( IF 4.5 ) Pub Date : 2020-08-22 , DOI: 10.1016/j.reactfunctpolym.2020.104716 Hang-Xin Zhu , Yun Liu , Yue-Xiao Wu , Jin-Jun Qiu , Cheng-Mei Liu
|
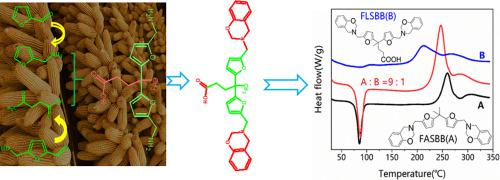
4,4-bis[5-(aminomethyl)furan-2-yl]pentanoic acid (BAFP), brand new renewable acid-containing diamines, was first utilized as feedstock for preparing fully biomass-based benzoxazine with self-catalytic characteristic. The resulting acid-containing bio-benzoxazine was prepared through Schiff base strategy. The onset ring-opening polymerization temperature of resulting benzoxazine is 173 °C determined by DSC, much lower than that of analogous bisfurfurylamine-based biobenzoxazine free of carboxylic group. The curing kinetics of as-prepared benzoxazine was probed by non-isothermal DSC method. The curing reaction consisted of three stages with overlapped regions with each other. The first stage was ascribed to initiation reaction triggered by protons of acid, with a activation energy of 116.9 KJ/mol calculated by Flynn-Wall-Ozawa method; the second stage was named combination-catalyzed stage for the fact that acid proton and phenolic proton was co-catalyzed the chain propagation, with a activation energy of 118.0 KJ/mol; The third stage was diffusion-controlled curing reaction with a activation energy of 137.3 KJ/mol. More interesting, the calculated apparent activation energy of three reactions decreased with the increase of monomer conversion.
中文翻译:

基于乙酰丙酸和糠胺的新型新型可再生含酸二胺衍生的独特自催化生物苯并恶嗪:合成,固化行为和性能
全新的含可再生酸的新型二胺4,4-双[5-(氨基甲基)呋喃-2-基]戊酸(BAFP)首先用作制备具有自催化特性的完全基于生物质的苯并恶嗪的原料。通过Schiff碱策略制备所得的含酸生物苯并恶嗪。通过DSC测定,所得苯并恶嗪的起始开环聚合温度为173℃,远低于不含羧基的类似基于双糠胺的生物苯并恶嗪的起始温度。通过非等温DSC方法研究了所制备的苯并恶嗪的固化动力学。固化反应包括彼此重叠的三个阶段。第一阶段归因于由酸质子触发的引发反应,其活化能通过Flynn-Wall-Ozawa方法计算为116.9 KJ / mol。由于酸质子和酚质子共催化链增长,活化能为118.0 KJ / mol,第二阶段被称为组合催化阶段。第三阶段是扩散控制的固化反应,活化能为137.3 KJ / mol。更有趣的是,三个反应的表观活化能随单体转化率的增加而降低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号