Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-08-22 , DOI: 10.1016/j.jmb.2020.08.015 Anwar Sadat 1 , Satyam Tiwari 1 , Kanika Verma 1 , Arjun Ray 2 , Mudassar Ali 3 , Vaibhav Upadhyay 4 , Anupam Singh 5 , Aseem Chaphalkar 1 , Asmita Ghosh 1 , Rahul Chakraborty 1 , Kausik Chakraborty 1 , Koyeli Mapa 6
|
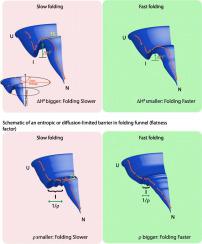
The folding landscape of proteins can change during evolution with the accumulation of mutations that may introduce entropic or enthalpic barriers in the protein folding pathway, making it a possible substrate of molecular chaperones in vivo. Can the nature of such physical barriers of folding dictate the feasibility of chaperone-assistance? To address this, we have simulated the evolutionary step to chaperone-dependence keeping GroEL/ES as the target chaperone and GFP as a model protein in an unbiased screen. We find that the mutation conferring GroEL/ES dependence in vivo and in vitro encode an entropic trap in the folding pathway rescued by the chaperonin. Additionally, GroEL/ES can edit the formation of non-native contacts similar to DnaK/J/E machinery. However, this capability is not utilized by the substrates in vivo. As a consequence, GroEL/ES caters to buffer mutations that predominantly cause entropic traps, despite possessing the capacity to edit both enthalpic and entropic traps in the folding pathway of the substrate protein.
中文翻译:

GROEL / ES在模型基板的演变过程中缓冲折叠路径中的熵陷阱。
蛋白质的折叠结构在进化过程中会随着突变的积累而发生变化,这些突变可能会在蛋白质折叠途径中引入熵或焓屏障,使其成为体内分子伴侣的可能底物。这种物理折叠障碍的性质可以决定伴侣伴侣辅助治疗的可行性吗?为了解决这个问题,我们模拟了进化伴侣伴侣的步骤,使GroEL / ES成为目标伴侣,而GFP作为模型蛋白在无偏性筛选中。我们发现该突变赋予体内和体外GroEL / ES依赖性在伴侣蛋白拯救的折叠途径中编码一个熵陷阱。此外,类似于DnaK / J / E机制,GroEL / ES可以编辑非本地联系人的形式。然而,该能力在体内没有被底物利用。结果,尽管具有在底物蛋白折叠途径中编辑焓和熵陷阱的能力,GroEL / ES仍能解决主要引起熵陷阱的缓冲突变。











































 京公网安备 11010802027423号
京公网安备 11010802027423号