Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Interfacial water and ion distribution determine ζ potential and binding affinity of nanoparticles to biomolecules.
Nanoscale ( IF 6.7 ) Pub Date : 2020-08-21 , DOI: 10.1039/d0nr03792c Dongyue Liang 1 , Udaya Dahal , Yongqian Kelly Zhang , Christian Lochbaum , Dhiman Ray , Robert J Hamers , Joel A Pedersen , Qiang Cui
Nanoscale ( IF 6.7 ) Pub Date : 2020-08-21 , DOI: 10.1039/d0nr03792c Dongyue Liang 1 , Udaya Dahal , Yongqian Kelly Zhang , Christian Lochbaum , Dhiman Ray , Robert J Hamers , Joel A Pedersen , Qiang Cui
Affiliation
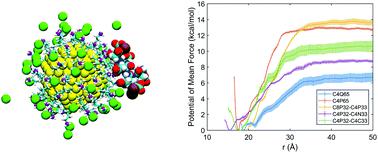
|
The molecular features that dictate interactions between functionalized nanoparticles and biomolecules are not well understood. This is in part because for highly charged nanoparticles in solution, establishing a clear connection between the molecular features of surface ligands and common experimental observables such as ζ potential requires going beyond the classical models based on continuum and mean field models. Motivated by these considerations, molecular dynamics simulations are used to probe the electrostatic properties of functionalized gold nanoparticles and their interaction with a charged peptide in salt solutions. Counterions are observed to screen the bare ligand charge to a significant degree even at the moderate salt concentration of 50 mM. As a result, the apparent charge density and ζ potential are largely insensitive to the bare ligand charge densities, which fall in the range of ligand densities typically measured experimentally for gold nanoparticles. While this screening effect was predicted by classical models such as the Manning condensation theory, the magnitudes of the apparent surface charge from microscopic simulations and mean-field models are significantly different. Moreover, our simulations found that the chemical features of the surface ligand (e.g., primary vs. quaternary amines, heterogeneous ligand lengths) modulate the interfacial ion and water distributions and therefore the interfacial potential. The importance of interfacial water is further highlighted by the observation that introducing a fraction of hydrophobic ligands enhances the strength of electrostatic binding of the charged peptide. Finally, the simulations highlight that the electric double layer is perturbed upon binding interactions. As a result, it is the bare charge density rather than the apparent charge density or ζ potential that better correlates with binding affinity of the nanoparticle to a charged peptide. Overall, our study highlights the importance of molecular features of the nanoparticle/water interface and underscores a set of design rules for the modulation of electrostatic driven interactions at nano/bio interfaces.
中文翻译:

界面水和离子的分布决定了纳米颗粒与生物分子的ζ电位和结合亲和力。
支配功能化的纳米粒子和生物分子之间相互作用的分子特征还不是很清楚。这部分是因为对于溶液中带高电荷的纳米颗粒,在表面配体的分子特征与常见实验可观察物(例如ζ电位)之间建立清晰的联系,需要超越基于连续谱和均值场模型的经典模型。基于这些考虑,分子动力学模拟被用来探测功能化金纳米粒子的静电特性以及它们与盐溶液中带电肽的相互作用。观察到抗衡离子即使在中等浓度的50 mM盐下也能显着筛选裸露的配体电荷。结果,表观电荷密度和ζ电势对裸露的配体电荷密度不敏感,裸子配体电荷密度落在通常通过实验对金纳米颗粒进行测量的配体密度范围内。尽管这种筛选效果是由经典模型(如曼宁凝聚理论)预测的,但从微观模拟和平均场模型得出的表观表面电荷的大小却存在显着差异。此外,我们的模拟发现表面配体的化学特征(例如,主要与主要季胺(异质配体长度)调节界面离子和水的分布,从而调节界面电位。界面水的重要性通过以下观察而进一步凸显:引入一部分疏水性配体可增强带电肽的静电结合强度。最后,模拟突出显示了双电层受到结合相互作用的干扰。结果,它是裸电荷密度而不是表观电荷密度或ζ与纳米颗粒与带电肽的结合亲和力更好相关的电位。总体而言,我们的研究突出了纳米颗粒/水界面分子特征的重要性,并强调了一组设计规则,用于调节纳米/生物界面上的静电驱动相互作用。
更新日期:2020-09-18
中文翻译:

界面水和离子的分布决定了纳米颗粒与生物分子的ζ电位和结合亲和力。
支配功能化的纳米粒子和生物分子之间相互作用的分子特征还不是很清楚。这部分是因为对于溶液中带高电荷的纳米颗粒,在表面配体的分子特征与常见实验可观察物(例如ζ电位)之间建立清晰的联系,需要超越基于连续谱和均值场模型的经典模型。基于这些考虑,分子动力学模拟被用来探测功能化金纳米粒子的静电特性以及它们与盐溶液中带电肽的相互作用。观察到抗衡离子即使在中等浓度的50 mM盐下也能显着筛选裸露的配体电荷。结果,表观电荷密度和ζ电势对裸露的配体电荷密度不敏感,裸子配体电荷密度落在通常通过实验对金纳米颗粒进行测量的配体密度范围内。尽管这种筛选效果是由经典模型(如曼宁凝聚理论)预测的,但从微观模拟和平均场模型得出的表观表面电荷的大小却存在显着差异。此外,我们的模拟发现表面配体的化学特征(例如,主要与主要季胺(异质配体长度)调节界面离子和水的分布,从而调节界面电位。界面水的重要性通过以下观察而进一步凸显:引入一部分疏水性配体可增强带电肽的静电结合强度。最后,模拟突出显示了双电层受到结合相互作用的干扰。结果,它是裸电荷密度而不是表观电荷密度或ζ与纳米颗粒与带电肽的结合亲和力更好相关的电位。总体而言,我们的研究突出了纳米颗粒/水界面分子特征的重要性,并强调了一组设计规则,用于调节纳米/生物界面上的静电驱动相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号