Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
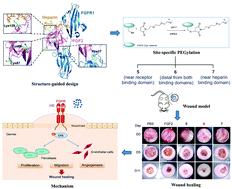
Structure-guided design, generation, and biofunction of PEGylated fibroblast growth factor 2 variants for wound healing.
Nanoscale ( IF 5.8 ) Pub Date : 2020-08-21 , DOI: 10.1039/d0nr05999d Jian Sun 1 , Jiamin Wu 1 , Hui Jin 2 , Te Ying 1 , Wei Jin 1 , Miaojuan Fan 1 , Jianhui Zhou 3 , Hui Chen 4 , Litai Jin 1 , Jie Zhou 1
Nanoscale ( IF 5.8 ) Pub Date : 2020-08-21 , DOI: 10.1039/d0nr05999d Jian Sun 1 , Jiamin Wu 1 , Hui Jin 2 , Te Ying 1 , Wei Jin 1 , Miaojuan Fan 1 , Jianhui Zhou 3 , Hui Chen 4 , Litai Jin 1 , Jie Zhou 1
Affiliation
|
Fibroblast growth factor 2 (FGF2) plays an important role in multiple physiological functions such as tissue repair. However, FGF2 has a short half-life in vivo due to protease degradation, thus limiting its clinical application. Traditional PEGylation has typically focused on the N-terminal α-amino group of FGF2. These modifications do not consider potential effects on protein function or structure, and sometimes lead to decreased bioactivity. In this study, we generated three PEGylated FGF2 variants based on the structure of the FGF2–FGFR–heparin ternary complex via gene mutation and PEGylation, and investigated the effects of these PEGylated sites on protein stability and bioactivity. Compared with native FGF2, all PEG–FGF2 conjugates exhibited significantly improved stability. Conjugates PEGylated at a site separated from both binding regions more effectively promoted proliferation, migration and angiogenesis than FGF2 in vitro, and exhibited excellent wound healing activity in vivo, making these conjugates potential therapeutic candidates for wound healing. Computer-assisted modification based on structure reveals the detailed structural characteristics of proteins, allowing efficient protein modification for improved stability and activity. This structure-guided PEGylation offers a more reliable modification strategy and should be applied for the rational design of protein-based therapeutics.
中文翻译:

用于伤口愈合的PEG化成纤维细胞生长因子2变体的结构指导设计,产生和生物功能。
成纤维细胞生长因子2(FGF2)在多种生理功能(例如组织修复)中起重要作用。然而,由于蛋白酶降解,FGF2在体内具有短的半衰期,因此限制了其临床应用。传统的聚乙二醇化通常集中在FGF2的N端α-氨基上。这些修饰没有考虑对蛋白质功能或结构的潜在影响,有时会导致生物活性降低。在这项研究中,我们产生3 PEG化的FGF2变体基础上的结构FGF2,FGFR-肝素三元复合物通过基因突变和聚乙二醇化,并研究了这些聚乙二醇化位点对蛋白质稳定性和生物活性的影响。与天然FGF2相比,所有PEG-FGF2共轭物均表现出明显改善的稳定性。与FGF2相比,在与两个结合区分开的位点处结合的PEG化比FGF2更有效地促进了增殖,迁移和血管生成,并且在体内表现出出色的伤口愈合活性,使这些结合物成为伤口愈合的潜在治疗候选物。基于结构的计算机辅助修饰揭示了蛋白质的详细结构特征,可以进行有效的蛋白质修饰以提高稳定性和活性。这种结构指导的聚乙二醇化提供了更可靠的修饰策略,应该用于基于蛋白质的治疗剂的合理设计。
更新日期:2020-09-18
中文翻译:

用于伤口愈合的PEG化成纤维细胞生长因子2变体的结构指导设计,产生和生物功能。
成纤维细胞生长因子2(FGF2)在多种生理功能(例如组织修复)中起重要作用。然而,由于蛋白酶降解,FGF2在体内具有短的半衰期,因此限制了其临床应用。传统的聚乙二醇化通常集中在FGF2的N端α-氨基上。这些修饰没有考虑对蛋白质功能或结构的潜在影响,有时会导致生物活性降低。在这项研究中,我们产生3 PEG化的FGF2变体基础上的结构FGF2,FGFR-肝素三元复合物通过基因突变和聚乙二醇化,并研究了这些聚乙二醇化位点对蛋白质稳定性和生物活性的影响。与天然FGF2相比,所有PEG-FGF2共轭物均表现出明显改善的稳定性。与FGF2相比,在与两个结合区分开的位点处结合的PEG化比FGF2更有效地促进了增殖,迁移和血管生成,并且在体内表现出出色的伤口愈合活性,使这些结合物成为伤口愈合的潜在治疗候选物。基于结构的计算机辅助修饰揭示了蛋白质的详细结构特征,可以进行有效的蛋白质修饰以提高稳定性和活性。这种结构指导的聚乙二醇化提供了更可靠的修饰策略,应该用于基于蛋白质的治疗剂的合理设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号