当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diversity-oriented synthesis of peptide-boronic acids by a versatile building-block approach
Chemical Science ( IF 7.6 ) Pub Date : 2020-08-21 , DOI: 10.1039/d0sc03999c Stefan P A Hinkes 1 , Severin Kämmerer 1 , Christian D P Klein 1
Chemical Science ( IF 7.6 ) Pub Date : 2020-08-21 , DOI: 10.1039/d0sc03999c Stefan P A Hinkes 1 , Severin Kämmerer 1 , Christian D P Klein 1
Affiliation
|
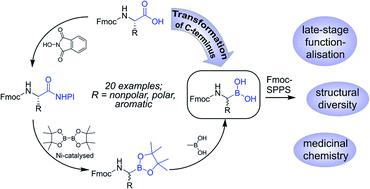
A new strategy for the synthesis of peptide-boronic acids (PBAs) is presented. 20 Fmoc-protected natural amino acids with orthogonal side-chain protection were straightforwardly converted into their corresponding boron analogues in three simple steps. Subsequent immobilisation on commercially available 1-glycerol polystyrene resin and on-resin transformations yielded a diversity of sequences in high purity. The strategy eliminates various synthetic obstacles such as multi-step routes, low yields, and inseparable impurities. The described method comprises great potential to be implemented in automated combinatorial approaches by markedly facilitating the access to a variety of PBAs. The coupling of amino acids or other building blocks with α-aminoboronates allows the creation of hybrid molecules with significant potential in various scientific disciplines, such as medicinal chemistry, structural biology, and materials science.
中文翻译:

通过多功能构建块方法以多样性为导向合成肽硼酸
提出了一种合成肽硼酸(PBA)的新策略。通过三个简单的步骤,将 20 种具有正交侧链保护的 Fmoc 保护的天然氨基酸直接转化为其相应的硼类似物。随后在市售 1-甘油聚苯乙烯树脂上的固定化和树脂上的转化产生了多种高纯度的序列。该策略消除了各种合成障碍,例如多步骤路线、低产率和不可分离的杂质。所描述的方法通过显着促进对各种 PBA 的访问,具有在自动化组合方法中实现的巨大潜力。氨基酸或其他结构单元与 α-氨基硼酸盐的偶联可以产生在药物化学、结构生物学和材料科学等各种科学学科中具有巨大潜力的杂化分子。
更新日期:2020-09-23
中文翻译:

通过多功能构建块方法以多样性为导向合成肽硼酸
提出了一种合成肽硼酸(PBA)的新策略。通过三个简单的步骤,将 20 种具有正交侧链保护的 Fmoc 保护的天然氨基酸直接转化为其相应的硼类似物。随后在市售 1-甘油聚苯乙烯树脂上的固定化和树脂上的转化产生了多种高纯度的序列。该策略消除了各种合成障碍,例如多步骤路线、低产率和不可分离的杂质。所描述的方法通过显着促进对各种 PBA 的访问,具有在自动化组合方法中实现的巨大潜力。氨基酸或其他结构单元与 α-氨基硼酸盐的偶联可以产生在药物化学、结构生物学和材料科学等各种科学学科中具有巨大潜力的杂化分子。









































 京公网安备 11010802027423号
京公网安备 11010802027423号