当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetics of the Reactions of Hydroxyl Radicals with Furan and Its Alkylated Derivatives 2-Methyl Furan and 2,5-Dimethyl Furan.
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2020-08-20 , DOI: 10.1021/acs.jpca.0c06321 Charlotte A Whelan 1 , Julia Eble 2 , Zara S Mir 1 , Mark A Blitz 1, 3 , Paul W Seakins 1 , Matthias Olzmann 2 , Daniel Stone 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2020-08-20 , DOI: 10.1021/acs.jpca.0c06321 Charlotte A Whelan 1 , Julia Eble 2 , Zara S Mir 1 , Mark A Blitz 1, 3 , Paul W Seakins 1 , Matthias Olzmann 2 , Daniel Stone 1
Affiliation
|
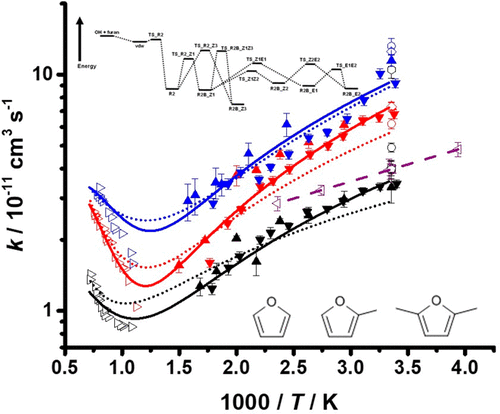
Furans are promising second generation biofuels with comparable energy densities to conventional fossil fuels. Combustion of furans is initiated and controlled to a large part by reactions with OH radicals, the kinetics of which are critical to understand the processes occurring under conditions relevant to low-temperature combustion. The reactions of OH radicals with furan (OH + F, R1), 2-methyl furan (OH + 2-MF, R2), and 2,5-dimethyl furan (OH + 2,5-DMF, R3) have been studied in this work over the temperature range 294–668 K at pressures between 5 mbar and 10 bar using laser flash photolysis coupled with laser-induced fluorescence (LIF) spectroscopy to generate and monitor OH radicals under pseudo-first-order conditions. Measurements at p ≤ 200 mbar were made in N2, using H2O2 or (CH3)3COOH radical precursors, while those at p ≥ 2 bar were made in He, using HNO3 as the radical precursor. The kinetics of reactions R1–R3 were observed to display a negative dependence on temperature over the range investigated, indicating the dominance of addition reactions under such conditions, with no significant dependence on pressure observed. Master equation calculations are in good agreement with the observed kinetics, and a combined parametrization of addition channels and abstraction channels for R1–R3 is provided on the basis of this work and previous shock tube measurements at higher temperatures. This work significantly extends the temperature range previously investigated for R1 and represents the first temperature-dependent measurements of R2 and R3 at temperatures relevant for atmospheric chemistry and low-temperature combustion.
中文翻译:

羟基自由基与呋喃及其烷基化衍生物2-甲基呋喃和2,5-二甲基呋喃的反应动力学。
呋喃有望成为第二代生物燃料,其能量密度可与传统化石燃料相媲美。呋喃的燃烧在很大程度上是通过与OH自由基的反应引发和控制的,其动力学对于了解在与低温燃烧有关的条件下发生的过程至关重要。研究了OH自由基与呋喃(OH + F,R1),2-甲基呋喃(OH + 2-MF,R2)和2,5-二甲基呋喃(OH + 2,5-DMF,R3)的反应在这项工作中,使用激光闪光光解结合激光诱导荧光(LIF)光谱在5 mbar至10 bar的压力下在294–668 K的温度范围内,在伪一级条件下生成和监测OH自由基。在测量p ≤200毫巴的N作了2,使用H2 Ò 2或(CH 3)3 COOH自由基前体,而在p ≥2巴在他进行了改造,用HNO 3作为自由基的前身。观察到反应R1-R3的动力学在所研究的范围内对温度呈负相关,表明在这种条件下加成反应占优势,而对压力没有显着依赖性。主方程计算与观察到的动力学非常吻合,并且在这项工作和以前在较高温度下的激波管测量结果的基础上,提供了R1-R3的附加通道和抽象通道的组合参数化。这项工作大大扩展了先前针对R1研究的温度范围,代表了在与大气化学和低温燃烧有关的温度下R2和R3的首次温度相关测量。
更新日期:2020-09-18
中文翻译:

羟基自由基与呋喃及其烷基化衍生物2-甲基呋喃和2,5-二甲基呋喃的反应动力学。
呋喃有望成为第二代生物燃料,其能量密度可与传统化石燃料相媲美。呋喃的燃烧在很大程度上是通过与OH自由基的反应引发和控制的,其动力学对于了解在与低温燃烧有关的条件下发生的过程至关重要。研究了OH自由基与呋喃(OH + F,R1),2-甲基呋喃(OH + 2-MF,R2)和2,5-二甲基呋喃(OH + 2,5-DMF,R3)的反应在这项工作中,使用激光闪光光解结合激光诱导荧光(LIF)光谱在5 mbar至10 bar的压力下在294–668 K的温度范围内,在伪一级条件下生成和监测OH自由基。在测量p ≤200毫巴的N作了2,使用H2 Ò 2或(CH 3)3 COOH自由基前体,而在p ≥2巴在他进行了改造,用HNO 3作为自由基的前身。观察到反应R1-R3的动力学在所研究的范围内对温度呈负相关,表明在这种条件下加成反应占优势,而对压力没有显着依赖性。主方程计算与观察到的动力学非常吻合,并且在这项工作和以前在较高温度下的激波管测量结果的基础上,提供了R1-R3的附加通道和抽象通道的组合参数化。这项工作大大扩展了先前针对R1研究的温度范围,代表了在与大气化学和低温燃烧有关的温度下R2和R3的首次温度相关测量。



























 京公网安备 11010802027423号
京公网安备 11010802027423号