当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics, Thermodynamics, and Scale-Up of an Azeotropic Drying Process: Mapping Rapid Phase Conversion with Process Analytical Technology
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-08-20 , DOI: 10.1021/acs.oprd.0c00275 Zachary E. X. Dance 1 , Morgan Crawford 2 , Aaron Moment 2 , Andrew Brunskill 3 , Busolo Wabuyele 4
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-08-20 , DOI: 10.1021/acs.oprd.0c00275 Zachary E. X. Dance 1 , Morgan Crawford 2 , Aaron Moment 2 , Andrew Brunskill 3 , Busolo Wabuyele 4
Affiliation
|
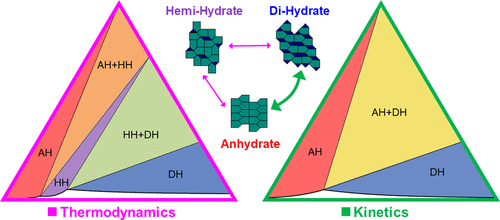
Distillation processes with several solid-state phases and dynamic multicomponent liquid-phase compositions can be challenging to understand and scale up because of complexity of the thermodynamics combined with process dynamics and kinetics. Often, development scientists will eschew the most efficient process because of the challenges in generating the necessary information and knowledge required to reproducibly implement it. Herein, we report the robust development of such a process: how it was characterized with in-line process analytical technology, off-line analytics, process modeling, and bench-top experiments and ultimately translated to a manufacturing scale. Through this exercise, we discovered a new solid phase and elucidated a canonical example of kinetic control and pseudoequilibrium. The detailed understanding of the thermodynamics and kinetics of the underlying physicochemical phenomena was used to define a simple control strategy that was implemented on an industrial scale to isolate the desired anhydrous crystal form. In this process, a crystalline dihydrate of a pharmaceutical intermediate was converted into an anhydrous form during distillation drying in a slurry. Water was removed by azeotropic distillation in acetonitrile at atmospheric pressure, while replenishing with dry acetonitrile. Below the critical water activity of conversion, the dihydrate was found to transform reliably and quickly into the anhydrate. Therefore, a concentrated slurry of the anhydrous form was obtained, which was telescoped into the subsequent, water-sensitive reaction. The discovery of an intermediate hemihydrate form added complexity to the system and presented a risk thermodynamically but was found to be kinetically difficult to access. In practice, the dihydrate rapidly converted directly to the anhydrate, and a molecular structural rationale for this behavior is proposed. The characterization and development of this process were enabled by in situ Raman spectroscopy and Fourier-transform infrared spectroscopy for determination of real-time solid-state form and solution water content, respectively. These tools allowed for construction of a process phase map as a function of temperature, water content, and slurry density while at the same time characterizing the kinetics of form transformations between dihydrate, hemihydrate, and anhydrous phases under relevant processing conditions. In addition, multiple off-line analytical techniques were utilized to fully characterize the solid phases, and ternary diagrams were used to explain the thermodynamics of the system and kinetic pathways. Based on this work, the process was transferred to an industrial scale and successfully executed using only a single off-line Karl Fischer measurement as a control strategy.
中文翻译:

共沸干燥过程的动力学,热力学和放大:利用过程分析技术绘制快速相变图
由于热力学的复杂性与过程动力学和动力学相结合,具有几种固态相和动态多组分液相组成的蒸馏过程难以理解和规模化。通常,开发科学家会避开最有效的过程,因为在生成可重复实施该过程所需的必要信息和知识方面存在挑战。本文中,我们报告了这种过程的强劲发展:如何通过在线过程分析技术,离线分析,过程建模和台式实验对其进行表征,并最终转化为制造规模。通过此练习,我们发现了一个新的固相,并阐明了动力学控制和假平衡的典范实例。对潜在物理化学现象的热力学和动力学的详细理解被用来定义一种简单的控制策略,该策略已在工业规模上实施以分离出所需的无水晶体形式。在该方法中,在将浆液蒸馏干燥期间,将药物中间体的结晶二水合物转化为无水形式。在大气压下通过在乙腈中共沸蒸馏除去水,同时补充干燥的乙腈。在转化的临界水活度以下,发现二水合物可靠,快速地转化为无水物。因此,获得了无水形式的浓缩浆液,将其通过套筒伸缩至随后的水敏反应中。中间半水合物形式的发现增加了系统的复杂性,并且在热力学上存在风险,但发现在动力学上难以接近。在实践中,二水合物迅速直接转化为无水物,并提出了该行为的分子结构原理。通过原位拉曼光谱和傅里叶变换红外光谱分别测定实时固态形式和溶液水含量,可以表征和开发该方法。这些工具允许根据温度,水含量和浆液密度构建工艺相图,同时表征相关工艺条件下二水合物,半水合物和无水相之间形态转化的动力学。此外,利用多种离线分析技术来完全表征固相,并使用三元图解释了系统的热力学和动力学路径。基于这项工作,该过程已转移到工业规模,并且仅使用单个离线Karl Fischer测量作为控制策略就成功地执行了该过程。
更新日期:2020-09-20
中文翻译:

共沸干燥过程的动力学,热力学和放大:利用过程分析技术绘制快速相变图
由于热力学的复杂性与过程动力学和动力学相结合,具有几种固态相和动态多组分液相组成的蒸馏过程难以理解和规模化。通常,开发科学家会避开最有效的过程,因为在生成可重复实施该过程所需的必要信息和知识方面存在挑战。本文中,我们报告了这种过程的强劲发展:如何通过在线过程分析技术,离线分析,过程建模和台式实验对其进行表征,并最终转化为制造规模。通过此练习,我们发现了一个新的固相,并阐明了动力学控制和假平衡的典范实例。对潜在物理化学现象的热力学和动力学的详细理解被用来定义一种简单的控制策略,该策略已在工业规模上实施以分离出所需的无水晶体形式。在该方法中,在将浆液蒸馏干燥期间,将药物中间体的结晶二水合物转化为无水形式。在大气压下通过在乙腈中共沸蒸馏除去水,同时补充干燥的乙腈。在转化的临界水活度以下,发现二水合物可靠,快速地转化为无水物。因此,获得了无水形式的浓缩浆液,将其通过套筒伸缩至随后的水敏反应中。中间半水合物形式的发现增加了系统的复杂性,并且在热力学上存在风险,但发现在动力学上难以接近。在实践中,二水合物迅速直接转化为无水物,并提出了该行为的分子结构原理。通过原位拉曼光谱和傅里叶变换红外光谱分别测定实时固态形式和溶液水含量,可以表征和开发该方法。这些工具允许根据温度,水含量和浆液密度构建工艺相图,同时表征相关工艺条件下二水合物,半水合物和无水相之间形态转化的动力学。此外,利用多种离线分析技术来完全表征固相,并使用三元图解释了系统的热力学和动力学路径。基于这项工作,该过程已转移到工业规模,并且仅使用单个离线Karl Fischer测量作为控制策略就成功地执行了该过程。









































 京公网安备 11010802027423号
京公网安备 11010802027423号