当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nanoparticles Presenting Potent TLR7/8 Agonists Enhance Anti-PD-L1 Immunotherapy in Cancer Treatment.
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-08-20 , DOI: 10.1021/acs.biomac.0c00812 Anton A A Smith 1 , Emily C Gale 2 , Gillie A Roth 3 , Caitlin L Maikawa 3 , Santiago Correa 1 , Anthony C Yu 1 , Eric A Appel 1, 3
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-08-20 , DOI: 10.1021/acs.biomac.0c00812 Anton A A Smith 1 , Emily C Gale 2 , Gillie A Roth 3 , Caitlin L Maikawa 3 , Santiago Correa 1 , Anthony C Yu 1 , Eric A Appel 1, 3
Affiliation
|
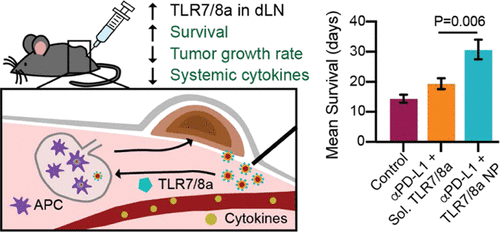
Cancer immunotherapy can be augmented with toll-like receptor agonist (TLRa) adjuvants, which interact with immune cells to elicit potent immune activation. Despite their potential, use of many TLRa compounds has been limited clinically due to their extreme potency and lack of pharmacokinetic control, causing systemic toxicity from unregulated systemic cytokine release. Herein, we overcome these shortcomings by generating poly(ethylene glycol)–poly(lactic acid) (PEG–PLA) nanoparticles (NPs) presenting potent TLR7/8a moieties on their surface. The NP platform allows precise control of TLR7/8a valency and resulting surface presentation through self-assembly using nanoprecipitation. We hypothesize that the pharmacokinetic profile of the NPs minimizes systemic toxicity, localizing TLR7/8a presentation to the tumor bed and tumor-draining lymph nodes. In conjunction with antiprogrammed death-ligand 1 (anti-PD-L1) checkpoint blockade, peritumoral injection of TLR7/8a NPs slows tumor growth, extends survival, and decreases systemic toxicity in comparison to the free TLR7/8a in a murine colon adenocarcinoma model. These NPs constitute a modular platform for controlling pharmacokinetics of immunostimulatory molecules, resulting in increased potency and decreased toxicity.
中文翻译:

呈现强效TLR7 / 8激动剂的纳米颗粒可增强抗PD-L1免疫疗法在癌症治疗中的作用。
Toll样受体激动剂(TLRa)佐剂可增强癌症免疫疗法,该佐剂与免疫细胞相互作用以引发有效的免疫激活。尽管具有潜力,但由于其极强的效力和缺乏药代动力学控制,许多TLRa化合物在临床上的使用受到了限制,导致不受控制的全身性细胞因子释放导致全身性毒性。在这里,我们通过产生在表面上具有强效TLR7 / 8a部分的聚(乙二醇)-聚(乳酸)(PEG-PLA)纳米颗粒(NPs)来克服这些缺点。NP平台可通过使用纳米沉淀进行自组装来精确控制TLR7 / 8a的化合价和产生的表面外观。我们假设NPs的药代动力学特征使全身毒性最小化,将TLR7 / 8a呈递定位于肿瘤床和引流淋巴结。与鼠结肠腺癌模型中的游离TLR7 / 8a相比,与游离的TLR7 / 8a相比,结合抗编程的死亡配体1(抗PD-L1)检查点封锁,肿瘤周围注射TLR7 / 8a NP可以减缓肿瘤生长,延长生存期并降低全身毒性。 。这些NP构成控制免疫刺激分子的药代动力学的模块平台,导致效力增加和毒性降低。
更新日期:2020-09-14
中文翻译:

呈现强效TLR7 / 8激动剂的纳米颗粒可增强抗PD-L1免疫疗法在癌症治疗中的作用。
Toll样受体激动剂(TLRa)佐剂可增强癌症免疫疗法,该佐剂与免疫细胞相互作用以引发有效的免疫激活。尽管具有潜力,但由于其极强的效力和缺乏药代动力学控制,许多TLRa化合物在临床上的使用受到了限制,导致不受控制的全身性细胞因子释放导致全身性毒性。在这里,我们通过产生在表面上具有强效TLR7 / 8a部分的聚(乙二醇)-聚(乳酸)(PEG-PLA)纳米颗粒(NPs)来克服这些缺点。NP平台可通过使用纳米沉淀进行自组装来精确控制TLR7 / 8a的化合价和产生的表面外观。我们假设NPs的药代动力学特征使全身毒性最小化,将TLR7 / 8a呈递定位于肿瘤床和引流淋巴结。与鼠结肠腺癌模型中的游离TLR7 / 8a相比,与游离的TLR7 / 8a相比,结合抗编程的死亡配体1(抗PD-L1)检查点封锁,肿瘤周围注射TLR7 / 8a NP可以减缓肿瘤生长,延长生存期并降低全身毒性。 。这些NP构成控制免疫刺激分子的药代动力学的模块平台,导致效力增加和毒性降低。









































 京公网安备 11010802027423号
京公网安备 11010802027423号