当前位置:
X-MOL 学术
›
ACS Comb. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Diversity-Oriented Synthesis of Thiazolidine-2-imines via Microwave-Assisted One-Pot, Telescopic Approach and Its Interaction with Biomacromolecules.
ACS Combinatorial Science ( IF 3.903 ) Pub Date : 2020-08-21 , DOI: 10.1021/acscombsci.0c00083 Ananya Anubhav Saikia 1 , Ramdas Nishanth Rao 1 , Barnali Maiti 1 , Musuvathi Motilal Balamurali 2 , Kaushik Chanda 1
ACS Combinatorial Science ( IF 3.903 ) Pub Date : 2020-08-21 , DOI: 10.1021/acscombsci.0c00083 Ananya Anubhav Saikia 1 , Ramdas Nishanth Rao 1 , Barnali Maiti 1 , Musuvathi Motilal Balamurali 2 , Kaushik Chanda 1
Affiliation
|
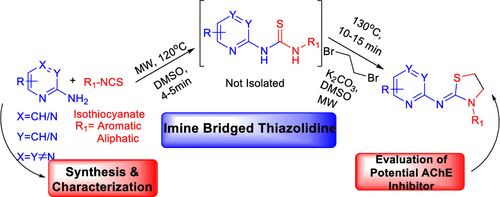
In this work, a one-pot, telescopic approach is described for the combinatorial library of thiazolidine-2-imines. The synthetic manipulation proceeds smoothly via the reaction of 2-aminopyridine/pyrazine/pyrimidine with substituted isothiocyanates followed by base catalyzed ring closure with 1,2-dibromoethane to obtain thiazolidine-2-imines with broad substrate scope and high functional group tolerance. The synthetic strategy merges well with the thiourea formation followed by base catalyzed ring closure reaction for the thiazolidine-2-imine synthesis in a more modular and straightforward approach. The synthetic procedure reported herein represents a cleaner route toward thiazolidine-2-imines as compared to traditional methodologies. Moreover, the biological significance of combinatorially synthesized thiazolidin-2-imines has been investigated for their use as possible inhibitors for acetyl cholinesterase through molecular docking studies.
中文翻译:

通过微波辅助的一锅、伸缩方法以多样性为导向合成噻唑烷-2-亚胺及其与生物大分子的相互作用。
在这项工作中,描述了一种用于 thiazolidine-2-亚胺组合库的单锅伸缩方法。通过2-氨基吡啶/吡嗪/嘧啶与取代的异硫氰酸酯反应,然后用1,2-二溴乙烷进行碱催化闭环,合成操作顺利进行,得到具有广泛底物范围和高官能团耐受性的噻唑烷-2-亚胺。合成策略与硫脲形成很好地结合,然后以更模块化和更直接的方法合成噻唑烷-2-亚胺的碱催化闭环反应。与传统方法相比,本文报道的合成程序代表了一种更清洁的 thiazolidine-2-亚胺路线。而且,
更新日期:2020-08-21
中文翻译:

通过微波辅助的一锅、伸缩方法以多样性为导向合成噻唑烷-2-亚胺及其与生物大分子的相互作用。
在这项工作中,描述了一种用于 thiazolidine-2-亚胺组合库的单锅伸缩方法。通过2-氨基吡啶/吡嗪/嘧啶与取代的异硫氰酸酯反应,然后用1,2-二溴乙烷进行碱催化闭环,合成操作顺利进行,得到具有广泛底物范围和高官能团耐受性的噻唑烷-2-亚胺。合成策略与硫脲形成很好地结合,然后以更模块化和更直接的方法合成噻唑烷-2-亚胺的碱催化闭环反应。与传统方法相比,本文报道的合成程序代表了一种更清洁的 thiazolidine-2-亚胺路线。而且,



























 京公网安备 11010802027423号
京公网安备 11010802027423号