当前位置:
X-MOL 学术
›
ChemPlusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Di(benzothienyl)cyclobutenes: Toward Strained Photoswitchable Fluorophores.
ChemPlusChem ( IF 3.0 ) Pub Date : 2020-08-21 , DOI: 10.1002/cplu.202000481 Dmytro Sysoiev 1 , Eliška Procházková 1 , Aleksander Semenenko 2 , Radek Pohl 1 , Svitlana Shishkina 2 , Blanka Klepetářová 1 , Volodymyr Shvadchak 1 , Dmytro A Yushchenko 1, 3
ChemPlusChem ( IF 3.0 ) Pub Date : 2020-08-21 , DOI: 10.1002/cplu.202000481 Dmytro Sysoiev 1 , Eliška Procházková 1 , Aleksander Semenenko 2 , Radek Pohl 1 , Svitlana Shishkina 2 , Blanka Klepetářová 1 , Volodymyr Shvadchak 1 , Dmytro A Yushchenko 1, 3
Affiliation
|
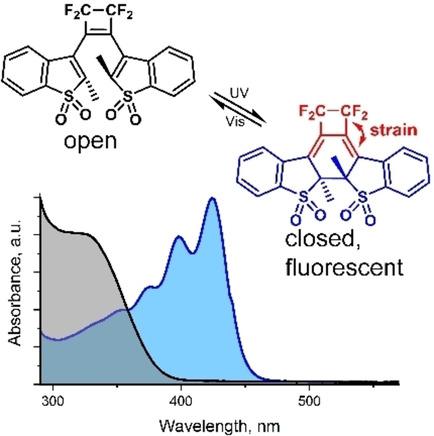
Bis(benzothienyl)ethene sulfones are very interesting molecules for super‐resolution microscopy due to their photoswitching properties. However, functionalization of the ‘classical’ bis(benzothienyl)ethene sulfones with a five‐membered central ring leads to significant decrease of quantum yields of photoconversion of the fluorescent closed form of the dye to the non‐fluorescent open form that limits their application in microscopy. Here, we designed and synthesized diarylethenes with a fluorinated four‐membered central ring that adds extra strain to the closed form of the dye. The reaction mechanism of their formation was studied, and byproducts formed upon structural rearrangement of the benzothiophene fragment were characterized. The photochromic properties of the new molecules were investigated by NMR and absorption spectroscopy. Some of these compounds show enhanced tendency to ring opening and have quantum yields of the ring‐opening reaction in the range of 0.2–0.5.
中文翻译:

二(苯并噻吩基)环丁烯:趋向应变的光开关荧光团。
双(苯并噻吩基)乙烯砜由于具有光开关特性,因此是用于超分辨率显微镜的非常有趣的分子。然而,具有五元中心环的“经典”双(苯并噻吩基)乙烯砜的功能化导致染料的荧光封闭形式向非荧光开放形式的光转化的量子产率显着降低,这限制了它们的应用。显微镜检查。在这里,我们设计并合成了带有氟化四元中心环的二芳烃,该四芳基环为染料的闭合形式增加了额外的应变。研究了它们形成的反应机理,并表征了苯并噻吩片段结构重排时形成的副产物。通过NMR和吸收光谱研究了新分子的光致变色性质。
更新日期:2020-09-16
中文翻译:

二(苯并噻吩基)环丁烯:趋向应变的光开关荧光团。
双(苯并噻吩基)乙烯砜由于具有光开关特性,因此是用于超分辨率显微镜的非常有趣的分子。然而,具有五元中心环的“经典”双(苯并噻吩基)乙烯砜的功能化导致染料的荧光封闭形式向非荧光开放形式的光转化的量子产率显着降低,这限制了它们的应用。显微镜检查。在这里,我们设计并合成了带有氟化四元中心环的二芳烃,该四芳基环为染料的闭合形式增加了额外的应变。研究了它们形成的反应机理,并表征了苯并噻吩片段结构重排时形成的副产物。通过NMR和吸收光谱研究了新分子的光致变色性质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号