Biochimie ( IF 3.3 ) Pub Date : 2020-08-21 , DOI: 10.1016/j.biochi.2020.07.013 Nathalie Pizzinat 1 , Varravaddheay Ong-Meang 1 , Florence Bourgailh-Tortosa 1 , Muriel Blanzat 2 , Lucie Perquis 2 , Daniel Cussac 1 , Angelo Parini 1 , Verena Poinsot 1
|
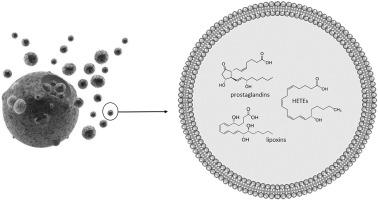
Recent works reported the relevance of cellular exosomes in the evolution of different pathologies. However, most of these studies focused on the ability of exosomes to convey mi-RNA from cell to cell. The level of knowledge concerning the transport of lipid mediators by these nanovesicles is more than fragmented. The role of lipid mediators in the inflammatory signaling is fairly well described, in particular concerning the derivatives of the arachidonic acid (AA), called eicosanoïds or lipid mediators. The aim of the present work was to study the transport of these lipids within the extracellular vesicles of rat bone marrow mesenchymal stem cells (BM-MSC) and the cardiomyoblast cell line H9c2, We were able to characterize, for the first time, complete profiles of oxilipins within these nanovesicles. We studied also the impact on these profiles, of the polyunsaturated fatty acids (PUFAs) know to be precursors of the inflammatory signaling molecules (AA, eicosapentaenoic acid EPA and Docosahexaenoic acid DHA), at physiological concentrations. By growing the progenitor cells under PUFAs supplementation, we provide a comprehensive assessment of the beneficial effect of ω‐3 PUFA therapy. Actually, our results tend to support the resolving role of the inflammation that stromal cell-derived extracellular vesicles can have within the cardiac microenvironment.
中文翻译:

MSC和心肌母细胞的细胞外囊泡是脂质介体的载体。
最近的工作报道了细胞外泌体在不同病理过程中的相关性。然而,这些研究大多数集中在外泌体将mi-RNA从细胞传递到细胞的能力。关于这些纳米囊泡转运脂质介体的知识水平远非零碎。脂质介体在炎症信号传导中的作用已得到很好的描述,特别是关于花生四烯酸(AA)的衍生物,称为二十碳五烯酸或脂质介体。本工作的目的是研究这些脂质在大鼠骨髓间充质干细胞(BM-MSC)和心肌成纤维细胞系H9c2的细胞外囊泡中的运输,我们能够首次表征完整的图谱。这些纳米囊泡中的oxilipins的数量。我们还研究了对这些配置文件的影响,在生理浓度下,已知多不饱和脂肪酸(PUFA)中的一部分是炎症信号分子(AA,二十碳五烯酸EPA和二十二碳六烯酸DHA)的前体。通过在补充PUFA的条件下培养祖细胞,我们对ω-3PUFA治疗的有益作用进行了全面评估。实际上,我们的结果倾向于支持炎症的解决作用,基质细胞衍生的细胞外囊泡在心脏微环境中可能具有炎症。











































 京公网安备 11010802027423号
京公网安备 11010802027423号