当前位置:
X-MOL 学术
›
Chem. Heterocycl. Comp.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
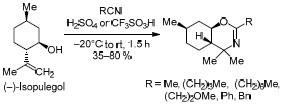
Synthesis of 1,3-Oxazine Derivatives Based on (–)-Isopulegol using the Ritter Reaction and Study of their Analgesic Activity
Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2020-08-21 , DOI: 10.1007/s10593-020-02753-x Nikolai S. Li-Zhulanov , Alla V. Pavlova , Dina V. Korchagina , Yurii V. Gatilov , Konstantin P. Volcho , Tatyana G. Tolstikova , Nariman F. Salakhutdinov

中文翻译:

Ritter反应合成基于(-)-异胡薄荷醇的1,3-恶嗪衍生物及其镇痛活性的研究

Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2020-08-21 , DOI: 10.1007/s10593-020-02753-x Nikolai S. Li-Zhulanov , Alla V. Pavlova , Dina V. Korchagina , Yurii V. Gatilov , Konstantin P. Volcho , Tatyana G. Tolstikova , Nariman F. Salakhutdinov
|

中文翻译:

Ritter反应合成基于(-)-异胡薄荷醇的1,3-恶嗪衍生物及其镇痛活性的研究











































 京公网安备 11010802027423号
京公网安备 11010802027423号