当前位置:
X-MOL 学术
›
Chem. Heterocycl. Comp.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
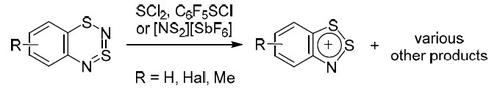
Interaction of 1,3λ 4 δ 2 ,2,4-benzodithiadiazines with neutral and charged S-electrophiles: SCl 2 , C 6 F 5 SCl, and NS 2 +
Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2020-08-21 , DOI: 10.1007/s10593-020-02760-y Alexander Yu. Makarov , Irina Yu. Bagryanskaya , Vladimir V. Zhivonitko

中文翻译:

1,3λ4δ2,2,4-苯并二噻二嗪与中性和带电S-亲电试剂的相互作用:SCl 2,C 6 F 5 SCl和NS 2 +

Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2020-08-21 , DOI: 10.1007/s10593-020-02760-y Alexander Yu. Makarov , Irina Yu. Bagryanskaya , Vladimir V. Zhivonitko
|

中文翻译:

1,3λ4δ2,2,4-苯并二噻二嗪与中性和带电S-亲电试剂的相互作用:SCl 2,C 6 F 5 SCl和NS 2 +












































 京公网安备 11010802027423号
京公网安备 11010802027423号