当前位置:
X-MOL 学术
›
Mol. Pharmacol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functional Impact of the G279S Substitution in the Adenosine A1-Receptor (A1R-G279S7.44), a Mutation Associated with Parkinson’s Disease
Molecular Pharmacology ( IF 3.2 ) Pub Date : 2020-09-01 , DOI: 10.1124/mol.120.000003 Shahrooz Nasrollahi-Shirazi , Daniel Szöllösi , Qiong Yang , Edin Muratspahic , Ali El-Kasaby , Sonja Sucic , Thomas Stockner , Christian Nanoff , Michael Freissmuth
Molecular Pharmacology ( IF 3.2 ) Pub Date : 2020-09-01 , DOI: 10.1124/mol.120.000003 Shahrooz Nasrollahi-Shirazi , Daniel Szöllösi , Qiong Yang , Edin Muratspahic , Ali El-Kasaby , Sonja Sucic , Thomas Stockner , Christian Nanoff , Michael Freissmuth
|
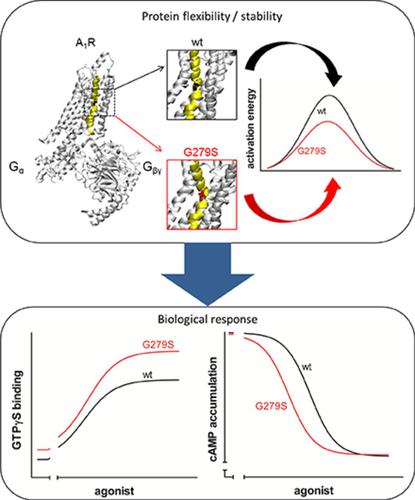
In medium-size, spiny striatal neurons of the direct pathway, dopamine D1- and adenosine A1-receptors are coexpressed and are mutually antagonistic. Recently, a mutation in the gene encoding the A1-receptor (A1R), A1R-G279S7.44, was identified in an Iranian family: two affected offspring suffered from early-onset l-DOPA–responsive Parkinson’s disease. The link between the mutation and the phenotype is unclear. Here, we explored the functional consequence of the G279S substitution on the activity of the A1-receptor after heterologous expression in HEK293 cells. The mutation did not affect surface expression and ligand binding but changed the susceptibility to heat denaturation: the thermodynamic stability of A1R-G279S7.44 was enhanced by about 2 and 8 K when compared with wild-type A1-receptor and A1R-Y288A7.53 (a folding-deficient variant used as a reference), respectively. In contrast, the kinetic stability was reduced, indicating a lower energy barrier for conformational transitions in A1R-G279S7.44 (73 ± 23 kJ/mol) than in wild-type A1R (135 ± 4 kJ/mol) or in A1R-Y288A7.53 (184 ± 24 kJ/mol). Consistent with this lower energy barrier, A1R-G279S7.44 was more effective in promoting guanine nucleotide exchange than wild-type A1R. We detected similar levels of complexes formed between D1-receptors and wild-type A1R or A1R-G279S7.44 by coimmunoprecipitation and bioluminescence resonance energy transfer. However, lower concentrations of agonist were required for half-maximum inhibition of dopamine-induced cAMP accumulation in cells coexpressing D1-receptor and A1R-G279S7.44 than in those coexpressing wild-type A1R. These observations predict enhanced inhibition of dopaminergic signaling by A1R-G279S7.44 in vivo, consistent with a pathogenic role in Parkinson’s disease.
中文翻译:

G279S取代在腺苷A1受体(A1R-G279S7.44)(与帕金森氏病有关的突变)中的功能影响
在直接通路的中型棘状纹状体神经元中,多巴胺D 1-和腺苷A 1-受体共表达且相互拮抗。最近,在一个伊朗家庭中发现了编码A 1受体(A 1 R)(A 1 R-G279S 7.44)的基因中的一个突变:两个受影响的后代患有早发性1 -DOPA反应性帕金森氏病。突变与表型之间的联系尚不清楚。在这里,我们探讨了G279S取代对A 1活性的功能影响HEK293细胞中异源表达后的β-受体。该突变不影响表面表达和配体结合,但改变了对热变性的敏感性:与野生型A 1受体和A 1 R相比,A 1 R-G279S 7.44的热力学稳定性提高了约2和8K。-Y288A 7.53(用作参考的折叠缺陷型)。相反,动力学稳定性降低了,表明与野生型A 1 R(135±4 kJ / mol)或在A 1 R-G279S 7.44(73±23 kJ / mol)中构象转变的能垒相比,较低一个1 R-Y288A 7.53(184±24kJ / mol)。与这种较低的能垒一致,A 1 R-G279S 7.44在促进鸟嘌呤核苷酸交换方面比野生型A 1 R更有效。我们检测到D 1受体与野生型A 1 R或A之间形成的复合物水平相似1 R-G279S 7.44通过共免疫沉淀和生物发光共振能量转移。然而,与共表达野生型A 1的细胞相比,在共表达D 1受体和A 1 R-G279S 7.44的细胞中半峰抑制多巴胺诱导的cAMP积累所需的激动剂浓度更低。R.这些观察结果预测A 1 R-G279S 7.44在体内对多巴胺能信号传导的抑制作用增强,这与帕金森氏病的致病作用一致。
更新日期:2020-08-20
中文翻译:

G279S取代在腺苷A1受体(A1R-G279S7.44)(与帕金森氏病有关的突变)中的功能影响
在直接通路的中型棘状纹状体神经元中,多巴胺D 1-和腺苷A 1-受体共表达且相互拮抗。最近,在一个伊朗家庭中发现了编码A 1受体(A 1 R)(A 1 R-G279S 7.44)的基因中的一个突变:两个受影响的后代患有早发性1 -DOPA反应性帕金森氏病。突变与表型之间的联系尚不清楚。在这里,我们探讨了G279S取代对A 1活性的功能影响HEK293细胞中异源表达后的β-受体。该突变不影响表面表达和配体结合,但改变了对热变性的敏感性:与野生型A 1受体和A 1 R相比,A 1 R-G279S 7.44的热力学稳定性提高了约2和8K。-Y288A 7.53(用作参考的折叠缺陷型)。相反,动力学稳定性降低了,表明与野生型A 1 R(135±4 kJ / mol)或在A 1 R-G279S 7.44(73±23 kJ / mol)中构象转变的能垒相比,较低一个1 R-Y288A 7.53(184±24kJ / mol)。与这种较低的能垒一致,A 1 R-G279S 7.44在促进鸟嘌呤核苷酸交换方面比野生型A 1 R更有效。我们检测到D 1受体与野生型A 1 R或A之间形成的复合物水平相似1 R-G279S 7.44通过共免疫沉淀和生物发光共振能量转移。然而,与共表达野生型A 1的细胞相比,在共表达D 1受体和A 1 R-G279S 7.44的细胞中半峰抑制多巴胺诱导的cAMP积累所需的激动剂浓度更低。R.这些观察结果预测A 1 R-G279S 7.44在体内对多巴胺能信号传导的抑制作用增强,这与帕金森氏病的致病作用一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号