当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric catalytic construction of fully substituted carbon stereocenters using acyclic α-branched β-ketocarbonyls: the “Methyl Rule” widely exists
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-08-20 , DOI: 10.1039/d0qo00673d Deqian Sun 1, 2, 3, 4, 5 , Shuang Yang 1, 2, 3, 4, 5 , Xinqiang Fang 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-08-20 , DOI: 10.1039/d0qo00673d Deqian Sun 1, 2, 3, 4, 5 , Shuang Yang 1, 2, 3, 4, 5 , Xinqiang Fang 1, 2, 3, 4, 5
Affiliation
|
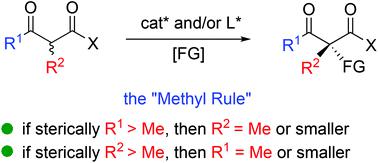
The catalytic asymmetric construction of tetrasubstituted carbon stereocenters constitutes one of the most challenging research topics in organic synthesis, which further plays vital roles in diverse fields including medicinal and materials chemistry. In this context, the α-functionalization of β-ketocarbonyl compounds serves as one of the most frequently used strategies to address the issue. Compared to cyclic β-ketocarbonyls, the catalytic asymmetric α-functionalization of acyclic β-ketocarbonyls is more difficult but has gained enough progress over the last several decades. This review illustrates the recent advances in this field, including asymmetric fluorination with acyclic α-branched β-ketocarbonyl participation, Michael addition, amination, aldol reaction, α-alkylation, α-alkynylation, α-oxygenation, Mannich reaction, etc. Furthermore, a thorough survey of all these reactions indicates the existence of a general principle which is called the “Methyl Rule”. Alternative methods that can complement the deficiency of reports of the use of direct asymmetric catalysis are also presented.
中文翻译:

使用无环α-支链β-酮羰基的不对称催化完全取代碳立体中心的构型:“甲基规则”广泛存在
四取代碳立体中心的催化不对称结构构成了有机合成中最具挑战性的研究主题之一,它在药物和材料化学等各个领域进一步发挥了至关重要的作用。在这种情况下,β-酮羰基化合物的α-官能化是解决该问题的最常用策略之一。与环状β-酮羰基相比,无环β-酮羰基的催化不对称α-官能化更加困难,但是在过去的几十年中已经获得了足够的进展。这篇综述说明了该领域的最新进展,包括不对称氟化与无环α-支化的β-酮羰基参与,迈克尔加成,胺化,醛醇反应,α-烷基化,α-炔基化,α-加氧,曼尼希反应,等。此外,对所有这些反应的彻底调查表明存在一个普遍的原则,即“甲基规则”。还提出了可以弥补使用直接不对称催化的报道不足的替代方法。
更新日期:2020-09-14
中文翻译:

使用无环α-支链β-酮羰基的不对称催化完全取代碳立体中心的构型:“甲基规则”广泛存在
四取代碳立体中心的催化不对称结构构成了有机合成中最具挑战性的研究主题之一,它在药物和材料化学等各个领域进一步发挥了至关重要的作用。在这种情况下,β-酮羰基化合物的α-官能化是解决该问题的最常用策略之一。与环状β-酮羰基相比,无环β-酮羰基的催化不对称α-官能化更加困难,但是在过去的几十年中已经获得了足够的进展。这篇综述说明了该领域的最新进展,包括不对称氟化与无环α-支化的β-酮羰基参与,迈克尔加成,胺化,醛醇反应,α-烷基化,α-炔基化,α-加氧,曼尼希反应,等。此外,对所有这些反应的彻底调查表明存在一个普遍的原则,即“甲基规则”。还提出了可以弥补使用直接不对称催化的报道不足的替代方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号