当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient transfer hydrogenation of carbonate salts from glycerol using water-soluble iridium N-heterocyclic carbene catalysts
Green Chemistry ( IF 9.3 ) Pub Date : 2020-08-20 , DOI: 10.1039/d0gc01958e Diana Ainembabazi 1, 2, 3, 4 , Kai Wang 1, 2, 3, 4 , Matthew Finn 1, 2, 3, 4 , James Ridenour 1, 2, 3, 4 , Adelina Voutchkova-Kostal 1, 2, 3, 4
Green Chemistry ( IF 9.3 ) Pub Date : 2020-08-20 , DOI: 10.1039/d0gc01958e Diana Ainembabazi 1, 2, 3, 4 , Kai Wang 1, 2, 3, 4 , Matthew Finn 1, 2, 3, 4 , James Ridenour 1, 2, 3, 4 , Adelina Voutchkova-Kostal 1, 2, 3, 4
Affiliation
|
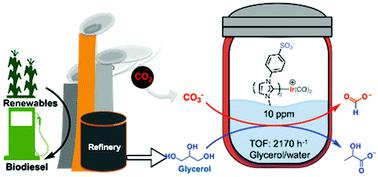
The transfer hydrogenation of CO2 and carbonates from biomass-derived alcohols, such as glycerol, to afford formic and lactic acid is a highly attractive path to valorizing two waste streams, and is significantly more thermodynamically favorable than direct carbonate hydrogenation. Expanding on our seminal report of the first homogeneous catalyst for this process, here we show that thermally-robust and water-soluble Ir(I) and Ir(III) N-heterocyclic carbene (NHC) complexes with sulfonate-functionalized wingtips are highly prolific and robust catalysts for carbonate transfer hydrogenation from glycerol, requiring no additives in aqueous media. The most prolific catalyst of the nine examined, [Ir(NHC-Ph-SO3−)2CO2]Na (cat 7), effectively facilitates the reaction at low catalyst loading (10 ppm) at 150 °C using microwave or conventional heating. The cation of the carbonate salt significantly impacts catalytic activity, with highest activity observed with Cs2CO3 (27 850 and 13 350 TONs for lactate and formate respectively in 6 hours, compared to 15 400 and 8120 with K2CO3). Catalytic amounts of Cs+ were found to significantly enhance activity with K2CO3. Catalyst 7 is even more prolific with conventional heating under a positive N2 pressure, reaching TOFs of >3000 h−1 and >2100 h−1 respectively for lactate and formate with K2CO3. The high activity of this catalyst compared to non-sulfonated and cyclooctadiene analogs is attributed to a combination of catalyst solubility in aqueous media and presence of π-acceptor carbonyl ligands. A catalytic mechanism is proposed for 7 involving O–H oxidative addition of glycerol, β-hydride elimination, bicarbonate dehydroxylation, insertion and reductive elimination.
中文翻译:

使用水溶性铱N-杂环卡宾催化剂从甘油高效转移加氢碳酸盐
从生物质衍生的醇(如甘油)转移CO 2和碳酸盐进行氢化,以生成甲酸和乳酸,这是使两种废物流均价化的极具吸引力的途径,并且比直接进行碳酸盐加氢在热力学上更为有利。在此方法的第一个均相催化剂的开创性报告的基础上,我们扩展了具有磺酸盐官能化的翼尖的耐热且水溶性的Ir(I)和Ir(III)N-杂环卡宾(NHC)配合物以及用于碳酸酯从甘油转移加氢的稳健催化剂,在水性介质中无需任何添加剂。九个最多产的催化剂检查,物[Ir(NHC-PH-SO 3 - )2使用微波或常规加热,CO 2 ] Na(催化剂7)可有效地促进在150°C的低催化剂负载量(10 ppm)下的反应。碳酸盐的阳离子显着影响催化活性,在Cs 2 CO 3中观察到最高的活性(乳酸和甲酸在6小时内分别为27 850和13 350 TON,而K 2 CO 3则为15 400和8120 )。发现催化量的Cs +可显着增强K 2 CO 3的活性。在N 2为正的情况下,常规加热下催化剂7的产量甚至更高乳酸和甲酸盐与K 2 CO 3的TOF分别达到> 3000 h -1和> 2100 h -1。与非磺化和环辛二烯类似物相比,该催化剂的高活性归因于催化剂在水性介质中的溶解度和π受体羰基配体的存在。提出了一种催化机制,涉及7种机制,包括O–H甘油的氧化加成,β-氢化物的消除,碳酸氢根的脱羟基作用,插入和还原性消除。
更新日期:2020-09-21
中文翻译:

使用水溶性铱N-杂环卡宾催化剂从甘油高效转移加氢碳酸盐
从生物质衍生的醇(如甘油)转移CO 2和碳酸盐进行氢化,以生成甲酸和乳酸,这是使两种废物流均价化的极具吸引力的途径,并且比直接进行碳酸盐加氢在热力学上更为有利。在此方法的第一个均相催化剂的开创性报告的基础上,我们扩展了具有磺酸盐官能化的翼尖的耐热且水溶性的Ir(I)和Ir(III)N-杂环卡宾(NHC)配合物以及用于碳酸酯从甘油转移加氢的稳健催化剂,在水性介质中无需任何添加剂。九个最多产的催化剂检查,物[Ir(NHC-PH-SO 3 - )2使用微波或常规加热,CO 2 ] Na(催化剂7)可有效地促进在150°C的低催化剂负载量(10 ppm)下的反应。碳酸盐的阳离子显着影响催化活性,在Cs 2 CO 3中观察到最高的活性(乳酸和甲酸在6小时内分别为27 850和13 350 TON,而K 2 CO 3则为15 400和8120 )。发现催化量的Cs +可显着增强K 2 CO 3的活性。在N 2为正的情况下,常规加热下催化剂7的产量甚至更高乳酸和甲酸盐与K 2 CO 3的TOF分别达到> 3000 h -1和> 2100 h -1。与非磺化和环辛二烯类似物相比,该催化剂的高活性归因于催化剂在水性介质中的溶解度和π受体羰基配体的存在。提出了一种催化机制,涉及7种机制,包括O–H甘油的氧化加成,β-氢化物的消除,碳酸氢根的脱羟基作用,插入和还原性消除。











































 京公网安备 11010802027423号
京公网安备 11010802027423号