当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
FXIIIa substrate peptide decorated BLZ945 nanoparticles for specifically remodeling tumor immunity
Biomaterials Science ( IF 5.8 ) Pub Date : 2020-08-20 , DOI: 10.1039/d0bm00713g Qi Wei 1, 2, 3, 4, 5 , Na Shen 1, 2, 3, 4, 5 , Haiyang Yu 1, 2, 3, 4, 5 , Yue Wang 1, 2, 3, 4, 5 , Zhaohui Tang 1, 2, 3, 4, 5 , Xuesi Chen 1, 2, 3, 4, 5
Biomaterials Science ( IF 5.8 ) Pub Date : 2020-08-20 , DOI: 10.1039/d0bm00713g Qi Wei 1, 2, 3, 4, 5 , Na Shen 1, 2, 3, 4, 5 , Haiyang Yu 1, 2, 3, 4, 5 , Yue Wang 1, 2, 3, 4, 5 , Zhaohui Tang 1, 2, 3, 4, 5 , Xuesi Chen 1, 2, 3, 4, 5
Affiliation
|
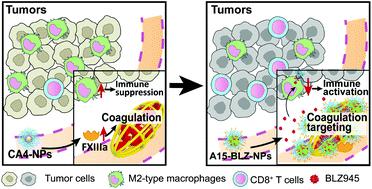
Combretastatin A4 nanoparticles (CA4-NPs), which notably inhibit tumor growth, were found to cause tumor regrowth due to the intratumoral enrichment of M2-type macrophages after treatment. Since BLZ945, an inhibitor of CSF-1 receptor (CSF-1R), depletes and inhibits the proliferation of M2-type macrophages, it has the potential to relieve the immunosuppressive microenvironment and improve anti-tumor therapy of CA4-NPs. However, CSF-1R exists widely, not only in macrophages, and BLZ945 could cause potential hepatotoxicity. It is necessary to establish a tumor-targeting drug delivery system to reduce the off-target and side effects of BLZ945. In this study, FXIIIa substrate peptide A15 decorated BLZ945 nanoparticles (A15-BLZ-NPs) were developed, in which, BLZ945-poly(D,L-lactide) (BLZ945-PLA), produced by ring-opening polymerization, was encapsulated in poly(ethylene glycol)-poly(D,L-lactide) (PEG5k-PLA5k), and A15 was decorated on the surface PEG segment. A15-BLZ-NPs could crosslink with fibrin through elevated FXIIIa and specifically target intratumoral coagulation spots induced by CA4-NPs. In vivo studies showed that CA4-NPs induced enhanced distribution of BLZ945 in tumors, as the BLZ945 content was 3.75-fold in the CA4-NP + A15-BLZ-NP group compared to that of A15-BLZ-NP single treatment. Meanwhile, compared to the CA4-NP group, the combination treatment significantly reduced the proportion of M2-type macrophages (from 64.4% to 24.5%) and enriched cytotoxic T lymphocytes (from 1.5% to 18.9%) in tumors, suggesting that A15-BLZ-NPs remodeled and activated tumor immunity after CA4-NP treatment. Furthermore, the combined treatment effectively improved the tumor inhibition rate to 73.4%, which was significantly higher than that of CA4-NP (15.5%) or A15-BLZ-NP (23.9%) single treatment. This work established a novel combination strategy for anti-tumor therapy.
中文翻译:

FXIIIa底物肽修饰的BLZ945纳米粒子可特异性重塑肿瘤免疫力
发现Combretastatin A4纳米颗粒(CA4-NPs)显着抑制肿瘤的生长,由于治疗后M2型巨噬细胞的肿瘤内富集,可导致肿瘤再生长。由于BLZ945是CSF-1受体(CSF-1R)的抑制剂,可耗尽并抑制M2型巨噬细胞的增殖,因此具有缓解免疫抑制微环境和改善CA4-NPs抗肿瘤治疗的潜力。然而,CSF-1R不仅广泛存在于巨噬细胞中,而且BLZ945可能引起潜在的肝毒性。必须建立一种靶向肿瘤的药物递送系统,以减少BLZ945的脱靶和副作用。在这项研究中,开发了FXIIIa底物肽A15装饰的BLZ945纳米颗粒(A15-BLZ-NPs),其中BLZ945-poly(D,L将通过开环聚合反应制得的-lactide)(BLZ945-PLA)封装在聚(乙二醇)-poly (D,L -lactide)(PEG 5k -PLA 5k)中,并在PEG片段的表面修饰A15 。A15-BLZ-NPs可以通过升高的FXIIIa与血纤蛋白交联,并特别靶向由CA4-NPs诱导的肿瘤内凝血点。体内研究表明,CA4-NPs诱导BLZ945在肿瘤中的分布增强,因为CA4-NP + A15-BLZ-NP组的BLZ945含量是A15-BLZ-NP单一治疗组的3.75倍。同时,与CA4-NP组相比,联合治疗可显着降低肿瘤中M2型巨噬细胞的比例(从64.4%降至24.5%)和富集细胞毒性T淋巴细胞(从1.5%至18.9%),提示A15-在CA4-NP治疗后,BLZ-NPs重塑并激活了肿瘤免疫力。此外,联合治疗有效地将肿瘤抑制率提高至73.4%,明显高于CA4-NP(15.5%)或A15-BLZ-NP(23.9%)的单药治疗。这项工作建立了一种抗肿瘤治疗的新型联合策略。
更新日期:2020-10-13
中文翻译:

FXIIIa底物肽修饰的BLZ945纳米粒子可特异性重塑肿瘤免疫力
发现Combretastatin A4纳米颗粒(CA4-NPs)显着抑制肿瘤的生长,由于治疗后M2型巨噬细胞的肿瘤内富集,可导致肿瘤再生长。由于BLZ945是CSF-1受体(CSF-1R)的抑制剂,可耗尽并抑制M2型巨噬细胞的增殖,因此具有缓解免疫抑制微环境和改善CA4-NPs抗肿瘤治疗的潜力。然而,CSF-1R不仅广泛存在于巨噬细胞中,而且BLZ945可能引起潜在的肝毒性。必须建立一种靶向肿瘤的药物递送系统,以减少BLZ945的脱靶和副作用。在这项研究中,开发了FXIIIa底物肽A15装饰的BLZ945纳米颗粒(A15-BLZ-NPs),其中BLZ945-poly(D,L将通过开环聚合反应制得的-lactide)(BLZ945-PLA)封装在聚(乙二醇)-poly (D,L -lactide)(PEG 5k -PLA 5k)中,并在PEG片段的表面修饰A15 。A15-BLZ-NPs可以通过升高的FXIIIa与血纤蛋白交联,并特别靶向由CA4-NPs诱导的肿瘤内凝血点。体内研究表明,CA4-NPs诱导BLZ945在肿瘤中的分布增强,因为CA4-NP + A15-BLZ-NP组的BLZ945含量是A15-BLZ-NP单一治疗组的3.75倍。同时,与CA4-NP组相比,联合治疗可显着降低肿瘤中M2型巨噬细胞的比例(从64.4%降至24.5%)和富集细胞毒性T淋巴细胞(从1.5%至18.9%),提示A15-在CA4-NP治疗后,BLZ-NPs重塑并激活了肿瘤免疫力。此外,联合治疗有效地将肿瘤抑制率提高至73.4%,明显高于CA4-NP(15.5%)或A15-BLZ-NP(23.9%)的单药治疗。这项工作建立了一种抗肿瘤治疗的新型联合策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号