当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On the Correlation between Pair Hydrophobicity and Mixing Enthalpies in Water-Alcohol Binary Mixtures.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-08-19 , DOI: 10.1021/acs.jpcb.0c05952 Ritaban Halder 1 , Biman Jana 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-08-19 , DOI: 10.1021/acs.jpcb.0c05952 Ritaban Halder 1 , Biman Jana 1
Affiliation
|
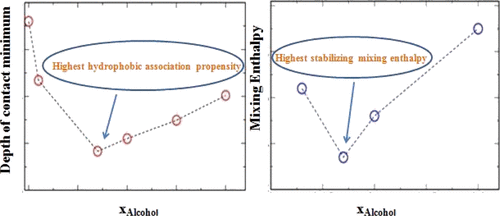
In this article, we have explored the extent of pair hydrophobicity in water–alcohol binary mixtures upon varying the chain length of the alcohol at several compositions. We have measured the pair hydrophobicity in water–methanol, water–propanol, and water–butanol mixtures. The pair hydrophobicity is measured by the depth of the first minimum (contact minimum) in the potential of mean force profile between a pair of neopentanes. In the case of water–methanol mixtures, the pair hydrophobicity is highest at xMeOH = 0.25, whereas in water–propanol mixtures, it is highest at xPrOH = 0.07, and in water–butanol mixture pair, hydrophobicity is highest at even lower alcohol concentration (xBuOH = 0.03). This indicates that as we increase the chain length of alcohol, the composition at which pair hydrophobicity is highest shifts toward a lower alcohol composition of the binary mixture. We have shown that the composition dependence of pair hydrophobicity echoes the trend observed in the calculated composition dependence of the enthalpy of mixing of the alcohol–water binary mixtures. The association pattern of the hydrophobic part of the alcohol also shows a change in trend around similar alcohol compositions. The hydrogen bond pattern around the alcohol rather than water exhibits a change in trend around those compositions. These results will improve our understanding of the composition-dependent phenomena of biomolecular processes in aqueous binary mixtures.
中文翻译:

水-醇二元混合物中配对疏水性与混合焓的相关性。
在本文中,我们研究了在几种成分下改变醇链长时,水-醇二元混合物中对疏水性的程度。我们测量了水-甲醇,水-丙醇和水-丁醇混合物中的对疏水性。该对疏水性通过一对新戊烷之间的平均力分布的电势中的第一个最小值(接触最小值)的深度来衡量。在水-甲醇混合物中,对疏水性在x MeOH = 0.25时最高,而在水-丙醇混合物中,对疏水性在x PrOH = 0.07时最高,而在水-丁醇混合物对中,疏水性最高,甚至更低酒精浓度(x BuOH= 0.03)。这表明随着我们增加醇的链长,对疏水性最高的组合物向二元混合物的较低醇组成转移。我们已经表明,成对疏水性的成分依赖性与在计算出的醇-水二元混合物的混合焓的成分依赖性中观察到的趋势相呼应。醇的疏水部分的缔合模式也显示相似醇组成周围的趋势变化。醇而不是水周围的氢键模式在那些组合物周围显示出趋势变化。这些结果将增进我们对水性二元混合物中生物分子过程的成分依赖性现象的理解。
更新日期:2020-09-18
中文翻译:

水-醇二元混合物中配对疏水性与混合焓的相关性。
在本文中,我们研究了在几种成分下改变醇链长时,水-醇二元混合物中对疏水性的程度。我们测量了水-甲醇,水-丙醇和水-丁醇混合物中的对疏水性。该对疏水性通过一对新戊烷之间的平均力分布的电势中的第一个最小值(接触最小值)的深度来衡量。在水-甲醇混合物中,对疏水性在x MeOH = 0.25时最高,而在水-丙醇混合物中,对疏水性在x PrOH = 0.07时最高,而在水-丁醇混合物对中,疏水性最高,甚至更低酒精浓度(x BuOH= 0.03)。这表明随着我们增加醇的链长,对疏水性最高的组合物向二元混合物的较低醇组成转移。我们已经表明,成对疏水性的成分依赖性与在计算出的醇-水二元混合物的混合焓的成分依赖性中观察到的趋势相呼应。醇的疏水部分的缔合模式也显示相似醇组成周围的趋势变化。醇而不是水周围的氢键模式在那些组合物周围显示出趋势变化。这些结果将增进我们对水性二元混合物中生物分子过程的成分依赖性现象的理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号