当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Toward a Scalable Synthesis and Process for EMA401, Part II: Development and Scale-Up of a Pyridine- and Piperidine-Free Knoevenagel–Doebner Condensation
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-08-20 , DOI: 10.1021/acs.oprd.0c00216 Eric Sidler 1 , Roger Humair 1 , Leo A. Hardegger 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-08-20 , DOI: 10.1021/acs.oprd.0c00216 Eric Sidler 1 , Roger Humair 1 , Leo A. Hardegger 1
Affiliation
|
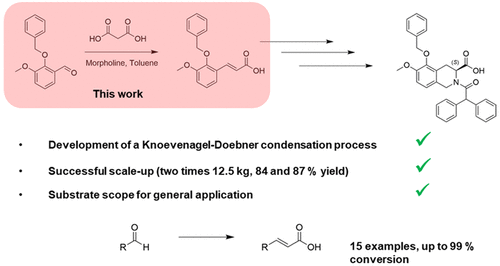
During route scouting for EMA401 (1), an angiotensin II type 2 antagonist, we identified the synthesis of key amino acid intermediate 2via its cinnamic acid derivative 3 as a streamlined option. In general, cinnamic acids can be synthesized from the corresponding aldehydes by a Knoevenagel–Doebner condensation in pyridine with piperidine as an organocatalyst. We aimed to replace both of these reagents and found novel conditions involving toluene as the solvent and morpholine as the organocatalyst. Scale-up of the process allowed the production of 25 kg of cinnamic acid 3 that was of the quality required for process development of the subsequent phenylalanine ammonia lyase-catalyzed step. The modified conditions were found to be widely applicable to alternative aldehydes and thus are of relevance to practitioners of chemical scale-up.
中文翻译:

迈向可扩展的EMA401合成和工艺,第二部分:无吡啶和无哌啶的Knoevenagel–Doebner缩合反应的开发和放大
在寻找EMA401(1)(一种血管紧张素II 2型拮抗剂)的途径时,我们确定了通过其肉桂酸衍生物3合成关键氨基酸中间体2的方法。通常,肉桂酸可以通过相应的醛类在Knoevenagel-Doebner缩合反应中在吡啶中以哌啶为有机催化剂而合成。我们旨在替换这两种试剂,并发现了涉及甲苯作为溶剂和吗啉作为有机催化剂的新条件。扩大生产规模可生产25公斤肉桂酸3这是后续苯丙氨酸氨裂合酶催化步骤的工艺开发所需的质量。发现修饰的条件广泛适用于替代醛,因此与化学大规模生产者有关。
更新日期:2020-09-20
中文翻译:

迈向可扩展的EMA401合成和工艺,第二部分:无吡啶和无哌啶的Knoevenagel–Doebner缩合反应的开发和放大
在寻找EMA401(1)(一种血管紧张素II 2型拮抗剂)的途径时,我们确定了通过其肉桂酸衍生物3合成关键氨基酸中间体2的方法。通常,肉桂酸可以通过相应的醛类在Knoevenagel-Doebner缩合反应中在吡啶中以哌啶为有机催化剂而合成。我们旨在替换这两种试剂,并发现了涉及甲苯作为溶剂和吗啉作为有机催化剂的新条件。扩大生产规模可生产25公斤肉桂酸3这是后续苯丙氨酸氨裂合酶催化步骤的工艺开发所需的质量。发现修饰的条件广泛适用于替代醛,因此与化学大规模生产者有关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号