当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Toward a Scalable Synthesis and Process for EMA401, Part III: Using an Engineered Phenylalanine Ammonia Lyase Enzyme to Synthesize a Non-natural Phenylalanine Derivative
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-08-20 , DOI: 10.1021/acs.oprd.0c00217 Leo A. Hardegger 1 , Pascal Beney 1 , Dominique Bixel 1 , Christian Fleury 1 , Feng Gao 2 , Alexandre Grand-Guillaume Perrenoud 1 , Xingxian Gu 2 , Julien Haber 1 , Tao Hong 2 , Roger Humair 1 , Andreas Kaegi 1 , Michael Kibiger 1 , Florian Kleinbeck 1 , Van Tong Luu 1 , Lukas Padeste 1 , Florian A. Rampf 1 , Thomas Ruch 1 , Thierry Schlama 1 , Eric Sidler 1 , Anikó Udvarhelyi 3 , Bernhard Wietfeld 1 , Yao Yang 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-08-20 , DOI: 10.1021/acs.oprd.0c00217 Leo A. Hardegger 1 , Pascal Beney 1 , Dominique Bixel 1 , Christian Fleury 1 , Feng Gao 2 , Alexandre Grand-Guillaume Perrenoud 1 , Xingxian Gu 2 , Julien Haber 1 , Tao Hong 2 , Roger Humair 1 , Andreas Kaegi 1 , Michael Kibiger 1 , Florian Kleinbeck 1 , Van Tong Luu 1 , Lukas Padeste 1 , Florian A. Rampf 1 , Thomas Ruch 1 , Thierry Schlama 1 , Eric Sidler 1 , Anikó Udvarhelyi 3 , Bernhard Wietfeld 1 , Yao Yang 2
Affiliation
|
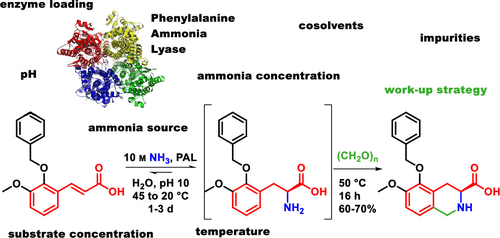
A process using an engineered phenylalanine ammonia lyase (PAL) enzyme was developed as part of an alternative route to a key intermediate of olodanrigan (EMA401). In the first part of this report, the detailed results from a screening for the optimal reaction conditions are presented, followed by a discussion of several workup strategies investigated. In the PAL-catalyzed reaction, 70–80% conversion of a cinnamic acid derivative to the corresponding phenylalanine derivative could be achieved. The phenylalanine derivative was subsequently telescoped to a Pictet–Spengler reaction with formaldehyde, and the corresponding tetrahydroisoquinoline derivative was isolated in 60–70% yield with >99.9:0.1 er. On the basis of our screenings, carbonate/carbamate-buffered ammonia at an NH3 concentration of 9–10 M and pH 9.5–10.5 was found to be optimal. Enzyme loadings down to 2.5 wt % (E:S = 1:40 w/w) could be achieved, and substrate concentrations between 3–9 v/w (1.17–0.39 M) were found to be compatible with the reaction conditions. A temperature gradient was applied in the final process: a pre-equilibrium was established at 45 °C, before making use of the temperature dependence of the entropy term with subsequent cooling to 20 °C to achieve maximum conversion. This temperature gradient also allowed balancing of the enzyme stability (low at 45 °C, high at 20 °C) with the activity (high at 45 °C, low at 20 °C) in order to achieve optimal conversion (low at 45 °C, high at 20 °C). From the various workup operations investigated, a sequence consisting of denaturation of the enzyme, NH3/CO2 removal by distillation, acidification, and telescoping to the subsequent Pictet–Spengler cyclization was our preferred approach. The process presented in this study is a more sustainable, shorter, and more cost-effective alternative to the previous process.
中文翻译:

迈向EMA401的可扩展合成和方法,第三部分:使用工程化的苯丙氨酸氨分解酶合成非天然的苯丙氨酸衍生物
开发了一种使用工程化的苯丙氨酸氨裂合酶(PAL)酶的方法,作为通往olodanrigan(EMA401)关键中间体的替代途径的一部分。在本报告的第一部分中,介绍了筛选最佳反应条件的详细结果,然后讨论了所研究的几种后处理策略。在PAL催化的反应中,肉桂酸衍生物向相应的苯丙氨酸衍生物的转化率可达到70-80%。随后将苯丙氨酸衍生物与甲醛进行伸缩反应,以进行Pictet-Spengler反应,并分离出相应的四氢异喹啉衍生物,收率60-70%,> 99.9:0.1 er。根据我们的筛选,碳酸盐/氨基甲酸酯缓冲的氨为NH 3发现最佳浓度为9-10 M,pH值为9.5-10.5。可以实现低至2.5 wt%的酶负载(E:S = 1:40 w / w),并且发现3–9 v / w(1.17–0.39 M)之间的底物浓度与反应条件兼容。在最后的过程中应用了温度梯度:在利用熵项的温度依赖性并随后冷却至20°C以实现最大转化率之前,在45°C时建立了预平衡。该温度梯度还可以平衡酶的稳定性(在45°C时较低,在20°C时较高)与活性(在45°C时较高,在20°C时较低)之间保持平衡,以实现最佳转化率(在45°C时较低) C,最高20°C)。从所研究的各种后处理操作中,发现了一个由变性酶NH 3 / CO组成的序列2通过蒸馏,酸化和伸缩进行随后的Pictet-Spengler环化反应的去除是我们的首选方法。本研究中提出的过程是以前过程的更可持续,更短且更具成本效益的替代方法。
更新日期:2020-09-20
中文翻译:

迈向EMA401的可扩展合成和方法,第三部分:使用工程化的苯丙氨酸氨分解酶合成非天然的苯丙氨酸衍生物
开发了一种使用工程化的苯丙氨酸氨裂合酶(PAL)酶的方法,作为通往olodanrigan(EMA401)关键中间体的替代途径的一部分。在本报告的第一部分中,介绍了筛选最佳反应条件的详细结果,然后讨论了所研究的几种后处理策略。在PAL催化的反应中,肉桂酸衍生物向相应的苯丙氨酸衍生物的转化率可达到70-80%。随后将苯丙氨酸衍生物与甲醛进行伸缩反应,以进行Pictet-Spengler反应,并分离出相应的四氢异喹啉衍生物,收率60-70%,> 99.9:0.1 er。根据我们的筛选,碳酸盐/氨基甲酸酯缓冲的氨为NH 3发现最佳浓度为9-10 M,pH值为9.5-10.5。可以实现低至2.5 wt%的酶负载(E:S = 1:40 w / w),并且发现3–9 v / w(1.17–0.39 M)之间的底物浓度与反应条件兼容。在最后的过程中应用了温度梯度:在利用熵项的温度依赖性并随后冷却至20°C以实现最大转化率之前,在45°C时建立了预平衡。该温度梯度还可以平衡酶的稳定性(在45°C时较低,在20°C时较高)与活性(在45°C时较高,在20°C时较低)之间保持平衡,以实现最佳转化率(在45°C时较低) C,最高20°C)。从所研究的各种后处理操作中,发现了一个由变性酶NH 3 / CO组成的序列2通过蒸馏,酸化和伸缩进行随后的Pictet-Spengler环化反应的去除是我们的首选方法。本研究中提出的过程是以前过程的更可持续,更短且更具成本效益的替代方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号