当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of polycyclic aromatic hydrocarbons and heteroatomic bridges (N, S, and O) on optoelectronic properties of 1,3,5‐triazine derivatives: A computational insight
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-08-20 , DOI: 10.1002/poc.4128 Vidya V.M. 1 , Prabhakar Chetti 1
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-08-20 , DOI: 10.1002/poc.4128 Vidya V.M. 1 , Prabhakar Chetti 1
Affiliation
|
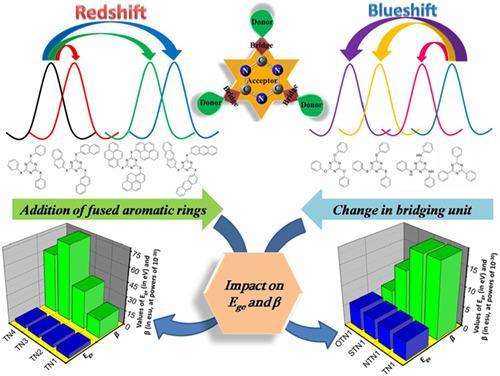
The theoretical investigation of four series of 1,3,5‐triazine derivatives (viz., TN, NTN, STN, and OTN series), which are substituted with polycyclic aromatic hydrocarbons, is carried out by employing density functional theory and time dependent‐density functional theory. The triazine ring acts as electron acceptor (A) and polycyclic aromatic hydrocarbons act as electron donors (D). The aromatic hydrocarbons like benzene, naphthalene (two fused rings), anthracene (three fused rings), and pyrene (four fused rings) are substituted on 1,3,5‐triazine core by means of a bridging heteroatom N, S, and O in the case of NTN, STN, and OTN series of molecules, respectively, whereas polycyclic aromatic hydrocarbons are directly connected to 1,3,5‐triazine in the case of TN series of molecules. The present work aims at examining the impact of number of fused aromatic rings and changing the bridging unit between electron acceptor and electron donor on absorption properties, HOMO‐LUMO gap and first hyperpolarizabilities of molecules under study and provides important insights for architecture of organic molecules for applications in organic optoelectronics.
中文翻译:

多环芳烃和杂原子桥(N,S和O)对1,3,5-三嗪衍生物的光电性能的影响:计算的见解
通过利用密度泛函理论和时间相关理论对四环1,3,5-三嗪衍生物(即TN,NTN,STN和OTN系列)进行了理论研究,这些衍生物被多环芳烃取代。密度泛函理论。三嗪环充当电子受体(A),多环芳烃充当电子供体(D)。芳烃如苯,萘(两个稠环),蒽(三个稠环)和pyr(四个稠环)通过杂原子N,S和O取代在1,3,5-三嗪核心上对于NTN,STN和分别为OTN系列分子,而在TN系列分子的情况下,多环芳烃直接与1,3,5-三嗪连接。本工作旨在研究稠合芳环数量的影响以及改变电子受体与电子给体之间的桥联单元对所研究分子的吸收特性,HOMO-LUMO间隙和第一超极化性的影响,并为有机分子的结构提供重要见解。在有机光电中的应用。
更新日期:2020-08-20
中文翻译:

多环芳烃和杂原子桥(N,S和O)对1,3,5-三嗪衍生物的光电性能的影响:计算的见解
通过利用密度泛函理论和时间相关理论对四环1,3,5-三嗪衍生物(即TN,NTN,STN和OTN系列)进行了理论研究,这些衍生物被多环芳烃取代。密度泛函理论。三嗪环充当电子受体(A),多环芳烃充当电子供体(D)。芳烃如苯,萘(两个稠环),蒽(三个稠环)和pyr(四个稠环)通过杂原子N,S和O取代在1,3,5-三嗪核心上对于NTN,STN和分别为OTN系列分子,而在TN系列分子的情况下,多环芳烃直接与1,3,5-三嗪连接。本工作旨在研究稠合芳环数量的影响以及改变电子受体与电子给体之间的桥联单元对所研究分子的吸收特性,HOMO-LUMO间隙和第一超极化性的影响,并为有机分子的结构提供重要见解。在有机光电中的应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号