当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, characterization, and in vitro cytotoxic activity evaluation of 1,2‐disubstituted benzimidazole compounds
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-08-13 , DOI: 10.1002/poc.4125 Senem Akkoç 1
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-08-13 , DOI: 10.1002/poc.4125 Senem Akkoç 1
Affiliation
|
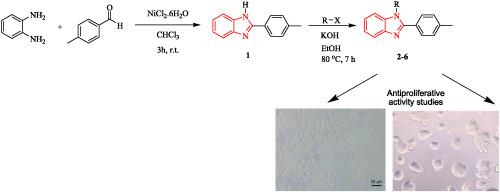
A series of 2‐p‐tolyl‐1H‐benzo[d]imidazole derivatives were synthesized and characterized. For finding an effective anticancer drug, which could be used in future generations, the developed heterocyclic compounds were screened in the human epithelial breast adenocarcinoma cell line (MCF‐7) and human liver epithelial hepatocellular carcinoma cell line (HepG2) using the MTT assay method. Two positive control drugs were used for comparison with the compounds. The substituents on the 1‐ and 2‐positions of the benzimidazole core had an important effect on the antiproliferation of cancerous cells. According to the results obtained, a compound, namely, 1‐(4‐methylbenzyl)‐2‐p‐tolyl‐1H‐benzo[d]imidazole, which has electron donating groups (CH3) in the para position of a phenyl ring, showed higher cytotoxic activities compared to other compounds towards liver and breast cell lines. The compounds were found to have more cytotoxicity in HepG2 rather than MCF‐7.
中文翻译:

1,2-二取代苯并咪唑化合物的设计,合成,表征和体外细胞毒性活性评估
合成并表征了一系列2 - p -tolyl- 1H-苯并[ d ]咪唑衍生物。为了找到可以在后代使用的有效抗癌药物,使用MTT分析法在人上皮性乳腺癌细胞系(MCF-7)和人肝上皮性肝癌细胞系(HepG2)中筛选了开发的杂环化合物。使用两种阳性对照药物与化合物进行比较。苯并咪唑核心1位和2位上的取代基对癌细胞的抗增殖具有重要作用。根据获得的结果,得到一种化合物,即1-(4-甲基苄基)-2-对甲苯基-1 H-苯并[ d在苯环对位具有给电子基团(CH 3)的对咪唑与其他化合物相比,对肝和乳腺癌细胞具有更高的细胞毒性。发现这些化合物在HepG2中比MCF-7具有更高的细胞毒性。
更新日期:2020-08-13
中文翻译:

1,2-二取代苯并咪唑化合物的设计,合成,表征和体外细胞毒性活性评估
合成并表征了一系列2 - p -tolyl- 1H-苯并[ d ]咪唑衍生物。为了找到可以在后代使用的有效抗癌药物,使用MTT分析法在人上皮性乳腺癌细胞系(MCF-7)和人肝上皮性肝癌细胞系(HepG2)中筛选了开发的杂环化合物。使用两种阳性对照药物与化合物进行比较。苯并咪唑核心1位和2位上的取代基对癌细胞的抗增殖具有重要作用。根据获得的结果,得到一种化合物,即1-(4-甲基苄基)-2-对甲苯基-1 H-苯并[ d在苯环对位具有给电子基团(CH 3)的对咪唑与其他化合物相比,对肝和乳腺癌细胞具有更高的细胞毒性。发现这些化合物在HepG2中比MCF-7具有更高的细胞毒性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号