当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of the functional groups amino and nitro on the reactivity of benzoxazoles and comparison with homologous benzothiazoles
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-08-03 , DOI: 10.1002/poc.4118 Ana L.R. Silva 1 , Maria D.M.C. Ribeiro da Silva 1
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-08-03 , DOI: 10.1002/poc.4118 Ana L.R. Silva 1 , Maria D.M.C. Ribeiro da Silva 1
Affiliation
|
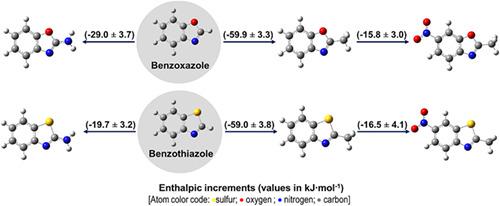
The energetic study of 2‐aminobenzoxazole (ABO) and 2‐methyl‐6‐nitrobenzoxazole (MNBO) has been developed using experimental and computational tools. The enthalpies of combustion, of fusion, and of sublimation of these compounds were measured by static‐bomb combustion calorimetry, differential scanning calorimetry, and Calvet microcalorimetry drop‐technique and/or the Knudsen‐effusion method. Additionally, we calculated the gas‐phase standard molar enthalpies of formation of these compounds, as well as of 2‐methyl‐6‐nitrobenzothiazole (MNBT), through high level ab initio calculations, at the G3(MP2)//B3LYP level of theory. Furthermore, the energetic effects associated with the presence of the amino and nitro groups on the core of benzoxazole or benzothiazole molecules were also evaluated, as well as stabilizing electronic interactions occurring in the molecules. The latter were investigated through Natural Bonding Orbital (NBO) of the corresponding wave functions. Finally, the thermodynamic stability of the titled compounds was evaluated and a comparison with their sulfur heteroanalogs was achieved. In the gaseous phase, the oxygen derivatives exhibit the lowest tendency to decompose into their constituent elements at standard conditions.
中文翻译:

氨基和硝基官能团对苯并恶唑反应性的影响及与同源苯并噻唑的比较
使用实验和计算工具进行了对2-氨基苯并恶唑(ABO)和2-甲基-6-硝基苯并恶唑(MNBO)的能量研究。通过静态炸弹燃烧量热法,差示扫描量热法和Calvet微量量热法滴定技术和/或Knudsen扩散法测量了这些化合物的燃烧,熔融和升华焓。此外,我们通过高水平的从头算计算得出了这些化合物以及2-甲基-6-硝基苯并噻唑(MNBT)的气相标准摩尔摩尔焓,其计算公式为G3(MP2)// B3LYP。理论。此外,还评估了与在苯并恶唑或苯并噻唑分子核心上存在氨基和硝基相关的能量效应,以及稳定分子中发生的电子相互作用。通过相应波函数的自然键合轨道(NBO)研究了后者。最后,评估了标题化合物的热力学稳定性,并与它们的硫杂类似物进行了比较。在气相中,氧衍生物在标准条件下表现出最低的分解成其组成元素的趋势。
更新日期:2020-08-03
中文翻译:

氨基和硝基官能团对苯并恶唑反应性的影响及与同源苯并噻唑的比较
使用实验和计算工具进行了对2-氨基苯并恶唑(ABO)和2-甲基-6-硝基苯并恶唑(MNBO)的能量研究。通过静态炸弹燃烧量热法,差示扫描量热法和Calvet微量量热法滴定技术和/或Knudsen扩散法测量了这些化合物的燃烧,熔融和升华焓。此外,我们通过高水平的从头算计算得出了这些化合物以及2-甲基-6-硝基苯并噻唑(MNBT)的气相标准摩尔摩尔焓,其计算公式为G3(MP2)// B3LYP。理论。此外,还评估了与在苯并恶唑或苯并噻唑分子核心上存在氨基和硝基相关的能量效应,以及稳定分子中发生的电子相互作用。通过相应波函数的自然键合轨道(NBO)研究了后者。最后,评估了标题化合物的热力学稳定性,并与它们的硫杂类似物进行了比较。在气相中,氧衍生物在标准条件下表现出最低的分解成其组成元素的趋势。











































 京公网安备 11010802027423号
京公网安备 11010802027423号