当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tautomeric and E‐Z equilibria of the herbicide clethodim in water and organic solvents: A nuclear magnetic resonance and theoretical study
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-07-19 , DOI: 10.1002/poc.4108 Maricel Caputo 1, 2 , Diego D. Colasurdo 1, 2 , Patricia E. Allegretti 1, 3 , Sergio L. Laurella 1, 3
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-07-19 , DOI: 10.1002/poc.4108 Maricel Caputo 1, 2 , Diego D. Colasurdo 1, 2 , Patricia E. Allegretti 1, 3 , Sergio L. Laurella 1, 3
Affiliation
|
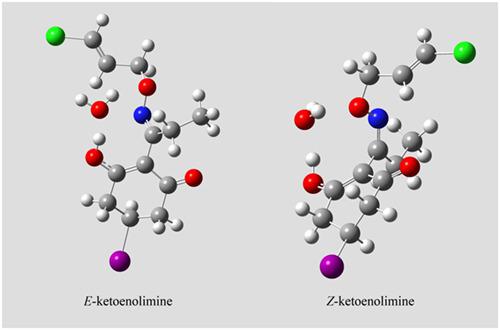
The herbicide clethodim can exist as different tautomeric forms and also as E‐Z isomers. Previous works have made some approach to the matter of tautomerization and have always stated that the E form was the only one stable. In this work, the investigation on the tautomeric equilibrium of clethodim is carried out in solution by means of proton nuclear magnetic resonance (1H NMR), carbon‐13 nuclear magnetic resonance (13C NMR), and correlation spectroscopy (COSY) experiments in four solvents (CDCl3, acetone‐d6, dimethylsulfoxide (DMSO)‐d6, and D2O). Major tautomeric species have been identified, and equilibrium constants have been calculated from the integration of hydrogens of the chloroallyl radical of clethodim. E‐ketoenolimine is the only detectable tautomer in CDCl3. Mixtures of E‐ketoenolimine and E‐diketoenamine tautomers are observed in acetone‐d6 and DMSO‐d6 (in ratios of 8:92 and 53:47, respectively). In water, the most abundant neutral tautomers are E‐ketoenolimine, E‐diketoenamine, and Z‐ketoenolimine (with a tautomeric ratio of 66:20:14). Identification of the E and Z tautomers is reported. Thermodynamic parameters (obtained by analysis of the effect of temperature) and theoretical calculations (energies and chemical shifts) support the assignations and tautomeric ratios when considering specific clethodim‐solvent hydrogen bonds. In the case of water, calculations suggest double bonded complexes that would be the cause of stability of the tautomers. These new observations on the structure of clethodim in solution open a new series of questions on its absorption mechanism, and they are also a tool for further chemical modification of this herbicide.
中文翻译:

水和有机溶剂中除草剂草胺定的互变异构和EZ平衡:核磁共振和理论研究
除草剂杀虫菊酯可以不同的互变异构形式存在,也可以作为E - Z异构体存在。以前的工作已经对互变异构问题进行了一些探讨,并始终指出E形式是唯一的一种稳定形式。在这项工作中,通过溶液中的质子核磁共振(1 H NMR),碳13核磁共振(13 C NMR)和相关光谱(COSY)实验研究了来昔定的互变异构平衡。四种溶剂(CDCl 3,丙酮d 6,二甲基亚砜(DMSO)d 6和D 2O)。已经确定了主要的互变异构物种,并且已经从头孢噻肟的氯烯丙基的氢的积分中计算出了平衡常数。E-酮烯胺是CDCl 3中唯一可检测的互变异构体。在丙酮d 6和DMSO d 6中观察到了E-酮烯胺和E-二酮烯胺互变异构体的混合物(比例分别为8:92和53:47)。在水中,最丰富的中性互变异构体是E-酮烯胺,E-二酮烯胺和Z-酮烯胺(互变异构比为66:20:14)。标识E和Z据报道互变异构体。当考虑特定的乙二胺-溶剂氢键时,热力学参数(通过温度影响的分析获得)和理论计算(能量和化学位移)支持赋值和互变异构比。对于水,计算表明双键复合物可能是互变异构体稳定性的原因。这些关于杀虫菊酯在溶液中的结构的新观察结果提出了一系列有关其吸收机理的问题,它们也是进一步对该除草剂进行化学修饰的工具。
更新日期:2020-07-19
中文翻译:

水和有机溶剂中除草剂草胺定的互变异构和EZ平衡:核磁共振和理论研究
除草剂杀虫菊酯可以不同的互变异构形式存在,也可以作为E - Z异构体存在。以前的工作已经对互变异构问题进行了一些探讨,并始终指出E形式是唯一的一种稳定形式。在这项工作中,通过溶液中的质子核磁共振(1 H NMR),碳13核磁共振(13 C NMR)和相关光谱(COSY)实验研究了来昔定的互变异构平衡。四种溶剂(CDCl 3,丙酮d 6,二甲基亚砜(DMSO)d 6和D 2O)。已经确定了主要的互变异构物种,并且已经从头孢噻肟的氯烯丙基的氢的积分中计算出了平衡常数。E-酮烯胺是CDCl 3中唯一可检测的互变异构体。在丙酮d 6和DMSO d 6中观察到了E-酮烯胺和E-二酮烯胺互变异构体的混合物(比例分别为8:92和53:47)。在水中,最丰富的中性互变异构体是E-酮烯胺,E-二酮烯胺和Z-酮烯胺(互变异构比为66:20:14)。标识E和Z据报道互变异构体。当考虑特定的乙二胺-溶剂氢键时,热力学参数(通过温度影响的分析获得)和理论计算(能量和化学位移)支持赋值和互变异构比。对于水,计算表明双键复合物可能是互变异构体稳定性的原因。这些关于杀虫菊酯在溶液中的结构的新观察结果提出了一系列有关其吸收机理的问题,它们也是进一步对该除草剂进行化学修饰的工具。









































 京公网安备 11010802027423号
京公网安备 11010802027423号