当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unveiling the high reactivity of strained dibenzocyclooctyne in [3 + 2] cycloaddition reactions with diazoalkanes through the molecular electron density theory
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-07-16 , DOI: 10.1002/poc.4100 Luis R. Domingo 1 , Nivedita Acharjee 2
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-07-16 , DOI: 10.1002/poc.4100 Luis R. Domingo 1 , Nivedita Acharjee 2
Affiliation
|
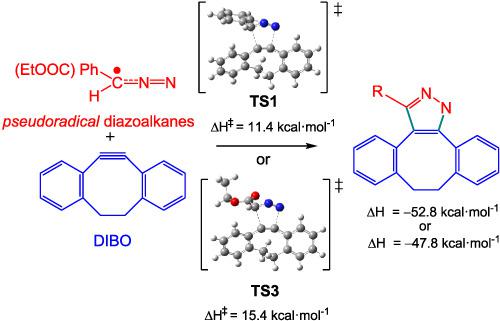
The strain‐promoted [3 + 2] cycloaddition (SP‐32CA) reactions of two diazoalkanes with strained dibenzocyclooctyne (DIBO) have been studied within the molecular electron density theory. Analysis of the electron localisation function of diazoalkanes allows characterising their pseudoradical structures. These pmr‐type 32CA reactions, which follow a one‐step mechanism with high asynchronicity and some polar character, have lower activation enthalpies, 11.4 and 15.4 kcal mol−1, than those with acetylene, 19.7 and 23.1 kcal mol−1, in agreement with the experimental outcomes. Analysis of the changes in electron density along the SP‐32CA reaction of phenyldiazomethane with DIBO indicates that the strain favours the depopulation of the C–C triple bond of DIBO, reducing the activation energy.
中文翻译:

通过分子电子密度理论揭示了在[3 + 2]与重氮烷烃的环加成反应中应变二苯并环辛炔的高反应活性
在分子电子密度理论中,已经研究了两种重氮烷与应变二苯并环辛炔(DIBO)的应变促进[3 + 2]环加成(SP-32CA)反应。重氮的电子定位功能的分析,可以表征其pseudoradical结构。这些pmr型32CA反应遵循具有高异步性和某些极性的一步机制,与乙炔,19.7和23.1 kcal mol -1相比,其活化焓较低,分别为11.4和15.4 kcal mol -1。,与实验结果一致。分析苯基重氮甲烷与DIBO的SP‐32CA反应过程中电子密度的变化表明,该菌株有利于DIBO的C–C三键的减少,从而降低了活化能。
更新日期:2020-07-16
中文翻译:

通过分子电子密度理论揭示了在[3 + 2]与重氮烷烃的环加成反应中应变二苯并环辛炔的高反应活性
在分子电子密度理论中,已经研究了两种重氮烷与应变二苯并环辛炔(DIBO)的应变促进[3 + 2]环加成(SP-32CA)反应。重氮的电子定位功能的分析,可以表征其pseudoradical结构。这些pmr型32CA反应遵循具有高异步性和某些极性的一步机制,与乙炔,19.7和23.1 kcal mol -1相比,其活化焓较低,分别为11.4和15.4 kcal mol -1。,与实验结果一致。分析苯基重氮甲烷与DIBO的SP‐32CA反应过程中电子密度的变化表明,该菌株有利于DIBO的C–C三键的减少,从而降低了活化能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号